Numerical investigation of the interaction between homogeneous and heterogeneous reactions of fuel-lean hydrogen–air mixtures over platinum in planar micro-channels
Abstract
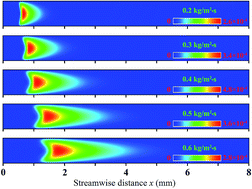
The interaction between homogeneous and heterogeneous reactions of a fuel-lean hydrogen–air mixture over platinum in a catalytic planar micro-channel was investigated numerically to assess the relative importance and respective contributions of homogeneous and heterogeneous reactions for different channel gap distances, inlet mass fluxes and equivalence ratios. Simulations were carried out with a two-dimensional computation that included detailed chemical kinetic mechanisms for the hetero-/homogeneous reaction. The results indicated that channel size can significantly affect the homogeneous reaction because of the effective radical and heat losses to the wall, despite the fact that the heterogeneous reaction remains basically unchanged. The catalytic wall helps in sustaining the homogeneous reaction in smaller channels by decreasing heat losses to the wall as a result of the exothermic heterogeneous reaction, while it also inhibits the homogeneous reaction because of wall radical quenching caused by typically higher absorption of OH radicals on the wall. Product species contribute even further to inhibiting homogeneous reaction because of the stronger diffusive mass flux in the smaller channel. In the present work, the main parameter effects on the interaction between homogeneous and heterogeneous reactions are explored. The limiting values of these parameters beyond which the homogeneous reaction becomes negligible as compared with the heterogeneous reaction are identified.

 Please wait while we load your content...
Please wait while we load your content...