Microwave mediated solvent free synthesis of formazans catalyzed by simple ionic liquids derived from dialkylammonium salts†
Pranab Jyoti Das* and
Jesmin Begum
Department of Chemistry, Gauhati University, Guwahati 781014, Assam, India. E-mail: pjd23@rediffmail.com
First published on 11th May 2015
Abstract
A microwave mediated, ionic liquid catalyzed, VOC free and one pot synthesis of formazans was developed. In an alternative procedure, resin immobilized diazonium ions was used as a solid supported reaction for formazan synthesis. The efficiency of both the procedures was examined with respect to yield of product, reduction of reaction time and environmental impact. Products were obtained in a short reaction time and in moderate to high yield. This study was undertaken to find an alternative green protocol for the synthesis of formazans using ionic liquid as catalyst in aqueous media in the absence of corrosive mineral acids, buffered solutions and VOCs.
Introduction
Formazans are a class of high nitrogen compounds first reported by Von Pechmann1 and by Bamberger.2 Several studies have been reported related to the establishment of their structure, photochemical transitions, tautomer formation and redox potential measurements.Formazans are characterized by their prominent π–π* transitions which are sensitive to substituents present in the phenyl rings, nature of the solvent and acidity and basicity of the medium.3–5
Formazan also find extensive use in analytical chemistry6,7 and their photochemical and thermochemical properties have been investigated.8 They are reported to possess a wide spectrum of biological activities such as antiviral,9,10 antimicrobial,11,12 anti inflammatory, analgesic13 and antifungal14 properties.
Synthetic methods for their preparation have been reviewed earlier.15 Their synthesis is based on two general procedures. The first is the reaction of aryldiazonium salts with phenylhydrazones of aldehydes in basic medium and the second is based on coupling of aryldiazonium ions with active methylene groups followed by Japp Klingemann rearrangement.16 However, these methods suffered from the fact that a variety of products are obtained depending on the temperature and basicity of the medium. These two parameters play key roles in the synthesis and requires fine control for obtaining good yields. Newer methods of synthesis are reported and notable among them are the use of solid–liquid phase transfer catalysts,17–20 liquid–liquid systems and crown ethers,21 methods reported by Tezcan et al.22–25 and a green method using the solid Lewis acid, BF3–SiO2.26 The commonly used method of synthesis involves three steps, the multiplicity of steps necessarily decreases the yield. Further since diazotization is the key step which requires the use of strong mineral acids and very low temperature, use of corrosive substances and temperature control cannot be avoided. In many diazocoupling reactions, acid catalyzed side reactions of the diazonium ions is usually observed further, acid sensitive substrates makes the synthesis even more cumbersome. Finally the use of bases to accelerate the reaction and the necessary use of organic solvents in the final reaction step between diazonium ions and the phenylhydrazone not only reduces yield due to insolubility of the diazonium ions in organic solvent but also makes the procedures unacceptable from the standpoint of green chemistry. While the only solvent free protocol reported by Bamoniri et al.27 was an improvement over other procedures however, the use of NaOH and BF3-etherate prompted us to look for better alternatives.
Ionic liquids are recognized as environmentally harmless media because of their low vapour pressure, high thermal and chemical stability and excellent solubilising characteristics. Their unique properties and the possibility of recovery for reuse resulted in their wide acceptance as media in many reactions and as catalysts as well. Reports are also available where ionic liquids have played dual role of a solvent and a catalyst. They are also known to influence the rate and selectivity of reactions. At present the domain of their application have widened attracting the attention of chemists with widely varied research interest.28–37 Among the range of ionic liquids, the salts of imidazolium, ammonium, thiazolium cations are especially popular and simple methods of their synthesis and characterization have been reported.38–42 Imidazolium based IL are reported to be biodegradable provided the N-alkyl group has more than four carbon atoms or has a hydroxyl groups.43 Their applicability in organic synthesis have been widely explored. However, the use of some of these IL is less attractive due to their high cost. Thus it is necessary to explore possibilities of preparing less expensive IL which may exhibit comparable, if not better, utility as catalyst and/or solvent.
Results and discussion
During the course of our study in developing cost effective ionic liquids for the synthesis of formazans, we prepared acidic ionic liquids from easily available dialkylamines and used them as catalyst for a microwave mediated four component reaction of aromatic aldehydes, phenylhydrazine aromatic amines and NaNO2 for the synthesis of formazans in appreciably reduced reaction time as well as in high yield. Three different ionic liquids, namely diethylammoniumhydrogensulphate, di-n-propylammoniumhydrogensulphate and di-n-butylammoniumhydrogensulphates were prepared by a reported procedure.47 Earlier results indicated a decrease in thermal stability according to the sequence [(n-propyl)2NH2]HSO4 > [Et2NH2]HSO4 > [(n-butyl)2NH2]HSO4.47 Since thermal stability of the ionic liquid did not play a significant part in the reaction being presently studied, we chose to use [Et2NH2]HSO4 in all the synthesis reported here. A trial reaction was examined using a mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar proportion of 4-chloroaniline, benzaldehyde, phenylhydrazine, 1.2 mole of NaNO2, 10 mol% of [Et2NH2]HSO4 and 1 mL of deionized water. The mixture was thoroughly ground to a homogeneous mixture. A deep red colour developed which lead us to believe that the four component reaction could be accomplished by grinding method only, however, work up of the product indicated that grinding at room temperature gave only about 30–40% yield. Having failed to obtain good yield, we exposed the well grounded mixture of the reactants in the same proportion to microwave irradiation (240 Watt) for 1.0 to 1.5 min and obtain the target product in 89% yield. Microwave irradiation at 240 Watt was found to be most suitable as increase in wattage resulting in charring of the reaction mixture leading to poor yield. To the best of our knowledge, acidic ionic liquids have never been used as catalyst for the synthesis formazans and hence this method assumes importance. The procedure was then generalized by using a wide variety of reactants and yields were found to be good and reaction time short. The yields of products were found to be independent of the nature of substituent groups in the carbonyl compounds and also in the aromatic amines used. Strict control of pH and temperature was unnecessary. Unlike previously reported procedure, this procedure is an excellent one pot four component synthesis of the formazan. The reaction is shown in Scheme 1.
1 molar proportion of 4-chloroaniline, benzaldehyde, phenylhydrazine, 1.2 mole of NaNO2, 10 mol% of [Et2NH2]HSO4 and 1 mL of deionized water. The mixture was thoroughly ground to a homogeneous mixture. A deep red colour developed which lead us to believe that the four component reaction could be accomplished by grinding method only, however, work up of the product indicated that grinding at room temperature gave only about 30–40% yield. Having failed to obtain good yield, we exposed the well grounded mixture of the reactants in the same proportion to microwave irradiation (240 Watt) for 1.0 to 1.5 min and obtain the target product in 89% yield. Microwave irradiation at 240 Watt was found to be most suitable as increase in wattage resulting in charring of the reaction mixture leading to poor yield. To the best of our knowledge, acidic ionic liquids have never been used as catalyst for the synthesis formazans and hence this method assumes importance. The procedure was then generalized by using a wide variety of reactants and yields were found to be good and reaction time short. The yields of products were found to be independent of the nature of substituent groups in the carbonyl compounds and also in the aromatic amines used. Strict control of pH and temperature was unnecessary. Unlike previously reported procedure, this procedure is an excellent one pot four component synthesis of the formazan. The reaction is shown in Scheme 1.
 | ||
| Scheme 1 One pot solvent free synthesis of formazans using acidic ionic liquids mediated by microwave. | ||
A proposed mechanism of the synthesis of formazan catalyzed by ionic liquid is shown in Scheme 2.
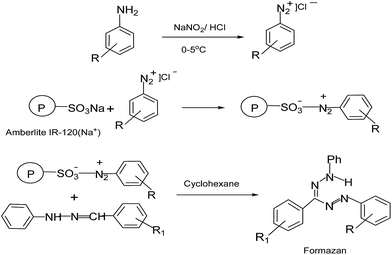
In addition to the use of IL as catalyst for the synthesis of formazans, we carried out a solid phase synthesis of formazans. In our earlier communications we had developed a method for immobilizing diazonium ions onto cation exchange resin with the aim of stabilizing them and used the diazonium ions successfully for the synthesis of triazines and the azo compounds.44–46 In this study, we extended the use of the immobilized diazonium ions for the synthesis of formazans in a solid phase reaction. In a typical solid phase procedure, the diazonium ions from 4-chloroaniline was prepared by the conventional method using mineral acid and NaNO2 at 0–5 °C and the corresponding diazonium ion obtained was immobilized on to an ion exchange resin namely Amberlite IR120 (Na+). Phenylhydrazones of benzaldehydes were prepared separately by established procedure,23 dissolved in cyclohexane and brought into reaction with the resin immobilized diazonium ions. The reaction was instantaneous as observed by the change in colour of the resin. The coloured resin was recovered, dried and the product 1-(4-chloro-phenyl)-3,5-diphenylformazan obtained by Soxhlet extraction of the resin beads using ethylacetate as the solvent. Reduced pressure removal of the solvent gave the products in moderate yield. The procedure was then generalized by using several combinations of different aldehydes and aromatic amines (Scheme 3).
The yield of the formazan have been found to be moderate in case of the solid phase synthetic procedure primarily because of strong adsorption of the dye to the resin which resulted in incomplete extraction with ethylacetate, whereas the IL catalyzed four component procedure described earlier gave excellent yield and recovery of the product was quantitative and easy. [Et2NH2]HSO4 being soluble in water could be removed completely from the products by several washings with distilled water. On comparing the two procedures used in this study it may further be mentioned that the use of IL resulted in a one pot solvent free synthesis while the solid phase technique involves three steps requiring preparations of the phenylhydrazone, preparation of diazonium ions using corrosive mineral acids before immobilization onto the ion exchange resin and finally the reaction of the immobilized diazonium ions with aldehyde–phenylhydrazone were carried out in an organic solvent. In both procedures, products were obtained in a pure form as indicated by TLC using prepared silicagel plates and ethylacetate: petroleum ether (40–60) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 as eluent and elaborate purification procedures were not necessary. The physical characteristics of the products and a comparison of the two techniques used is summarized in Table 1.
9 as eluent and elaborate purification procedures were not necessary. The physical characteristics of the products and a comparison of the two techniques used is summarized in Table 1.
| Entry | R | R1 | Yield (%) in | Mp °C | ||
|---|---|---|---|---|---|---|
| A* | B* | Obs | Lit | |||
| a * Reaction A in immobilized diazonium ions, Reaction B in ionic liquid, N stands for new compound. | ||||||
| 1 | H | H | 77 | 82 | 173–174 | 172–174 (ref. 21) |
| 2 | H | 4-OCH3 | 69 | 84 | 160–61 | 157–59 (ref. 21) |
| 3 | H | 4-NO2 | 70 | 81 | 199 | 196–98 (ref. 27) |
| 4 | H | 4-CH3 | 66 | 78 | 157 | 153–155 (ref. 27) |
| 5 | 2-Cl | H | 62 | 81 | 146 | 142–143 (ref. 23) |
| 6 | 3-Cl | H | 72 | 89 | 157–58 | 158 (ref. 23) |
| 7 | 4-Br | H | 70 | 83 | 190–91 | 189–190 (ref. 23) |
| 8 | 4-Cl | H | 65 | 89 | 120–121 | 119 (ref. 23) |
| 9 | 4-NO2 | H | 76 | 90 | 234–235 | N |
| 10 | 3-NO2 | H | 72 | 87 | 176 | N |
| 11 | 2-NO2 | H | 88 | 80 | 174–175 | N |
| 12 | 2,4-DiCl | H | 70 | 86 | 205 | N |
| 13 | 4-CH3CO | H | 80 | 90 | 214–216 | N |
| 14 | 4-CH3 | H | 68 | 85 | 153–154 | N |
| 15 | 4-Cl | 4-CH3 | 77 | 82 | 179–183 | N |
| 16 | 4-Cl | 2-CH3O | 72 | 83 | 180–181 | N |
| 17 | 4-CH3O | 2-Cl | 73 | 75 | 203–206 | N |
| 18 | 4-CH3 | 4-NO2 | 70 | 80 | 188–190 | N |
| 19 | 4-NO2 | 4-NO2 | 90 | 90 | 209 | N |
A comparative study of the present methods using IL and the solid phase synthesis to a few other procedures reported in literature is summarized in Table 2. Comparison reveals the superiority and better environmental acceptability of the one pot solvent free synthesis of formazans using cost effective and easily available Ionic liquids.
| Entry | Reagents | Yield (%) | Reaction | Obs | |
|---|---|---|---|---|---|
| Time | Steps | ||||
| 1 | PTC/M2CO3/CH2Cl2, M = K, Na | 45–70% | 1–4 h | 3 | VOC used21 |
| 2 | BF3–SiO2 | 78–88% | 1–2 min | 2 | Solvent free27 |
| 3 | Mineral acid, NaOH, CH3COONa | 80% | 3.5 h | 3 | VOC used23 |
| 4 | Alkali | 54–75% | — | 3 | Temp and pH control26 |
| 5 | Amberlite IR120 (Na+), acid, cyclohexane | 65–74% | 1–2 h | 3 | This work VOC used |
| 6 | Ionic liquid [Et2NH]HSO4 | 82–90% | 1–2 min | 1 | This work solvent free |
All formazans synthesized gave characteristics π–π* absorption in the visible region at 350 to 450 nm which on oxidation with dil. HNO3 at room temperature or with 5% KMnO4 solution, shifted the absorption maxima to around 300 nm indicating their conversion to tetrazolium salts.48 This was a confirmative test for the formation of formazans as the product. UV absorption of selected formazans before and after oxidation shown in Fig. 1 and 2a and b respectively.
 | ||
| Fig. 2 (a and b): UV spectra of tetrazolium salt of 1-(4-acetylphenyl)-3,5-diphenylformazan and 1-(4-chloro-2-nitrophenyl)-3-(4-nitrophenyl)-5-phenylformazan. | ||
Experimental
Chemicals were purchased from Loba chieme (India) and purified by procedures reported in literature.49 Acidic ionic liquids prepared from dialkylamines by a procedure reported earlier.47 Formazans obtained were confirmed by comparing their melting points with those reported in literature. Melting points were recorded in open capillaries and are uncorrected. Products were purified by repeated column chromatography. All new products were characterized by spectroscopic methods. UV-vis spectra were recorded in UV-1800 Shimadzu UV spectrophotometer, IR spectra were recorded in KBr pellets in a Perkin Elmer FT-IR 1600 spectrophotometer and 1H and 13C NMR were recorded in Bruker Bio Spin 300 MHz spectrometer using CDCl3 as solvent and TMS as internal standard. Mass spectra of new compounds were recorded in Micromass QTOF ESI-MS instrument (model HAB273) and microwave irradiation of reaction mixture was performed in reactor procured from Catalyst™(India).Preparation of ionic liquids: ionic liquids of dialkylamines
[Et2NH2]HSO4, [(n-propyl)2NH2]HSO4 and [(n-isopropyl)2NH2]HSO4 were prepared by a procedure describe earlier.47General procedure for synthesis of formazan using ionic liquid
A mixture of aromatic aldehyde (1 mmol), phenylhydrazine (1 mmol), aromatic amine (1 mmol) and NaNO2 (1.2 mmol), 10 mol% of [Et2NH2]HSO4 and 1 mL of deionized water was ground to a homogeneous mixture. The bright coloured mixture was exposed to microwave irradiation (240 Watt) for a 1–1.5 min. After completion of the reaction, the solid mass obtained was washed with water till free of ionic liquid. The remaining solid was dissolved in ethylacetate, dried using anhydrous Na2SO4 and product obtained by reduced pressure evaporation of the solvent. Finally the products were recrystallized from 95% (v/v) EtOH.General method for synthesis of formazan in solid phase
Resin immobilized diazonium ions was prepared by a procedure described earlier, recovered and dried.44–46 The phenylhydrazone of the aromatic aldehydes were prepared by reported methods.23 The hydrazones (1 mmol) was dissolved in 50 mL of cyclohexane and the solution percolated through a column slurry packed with 15 g of immobilized diazonium ions. On formation of the formazan dyes, the beads turned deep red. The beads were recovered, dried at room temperature and the product obtained by extraction of the beads with ethylacetate in a Soxhlet extraction apparatus. The products were tested for their purity by TLC using prepared silica gel plates and ethylacetate: petroleum ether (40–60) in the ration 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 as the eluent. TLC indicated purity of the product. Reduced pressure evaporation of the products gave the formazans.
9 as the eluent. TLC indicated purity of the product. Reduced pressure evaporation of the products gave the formazans.
Experimental data of selected products
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1556 (–NO2), 1415 (–N
C–), 1556 (–NO2), 1415 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1303 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.979 (1H, d, J = 7.6 Hz, NH), 7.140–8.496 (m, 14H, Ar–H), 13C NMR (75 MHz, CDCl3): δ ppm 114.72, 118.28, 120.43, 121.9, 123.48, 125.18, 127.99, 128.57, 132.37, 133.58, 134.31, 145.19, 150.01 HRMS (ESI): 344.612 calc. 243. HRMS (ESI): 346.128 (M+) calc. 345.35.
N–), 1303 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.979 (1H, d, J = 7.6 Hz, NH), 7.140–8.496 (m, 14H, Ar–H), 13C NMR (75 MHz, CDCl3): δ ppm 114.72, 118.28, 120.43, 121.9, 123.48, 125.18, 127.99, 128.57, 132.37, 133.58, 134.31, 145.19, 150.01 HRMS (ESI): 344.612 calc. 243. HRMS (ESI): 346.128 (M+) calc. 345.35.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1548 (–NO2), 1417 (–N
C–), 1548 (–NO2), 1417 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1302 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 7.227 (1H, s, N–H), 7.253–8.025 (14H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 112.30, 112.30, 113.24, 114.49, 116.98, 119.00, 126.52, 128.73, 129.19, 129.93, 138.44, 139.52, 143.58, 145.19, 145.9, HRMS (ESI): 346.128 (M+) calc. 345.35.
N–), 1302 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 7.227 (1H, s, N–H), 7.253–8.025 (14H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 112.30, 112.30, 113.24, 114.49, 116.98, 119.00, 126.52, 128.73, 129.19, 129.93, 138.44, 139.52, 143.58, 145.19, 145.9, HRMS (ESI): 346.128 (M+) calc. 345.35.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1555 (–NO2), 1419 (–N
C–), 1555 (–NO2), 1419 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1302 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.883 (1H, t, J = 7.8, J = 7.5, NH), 7.759–8.203 (14H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm. 116.47, 118.49, 121.01, 126.19, 127.25, 129.02, 130.04, 134.49, 136.34, 137.02, 142.16, 143.9, 147.84, 149.34, HRMS (ESI): 344.612 obs, 345.35 calc.
N–), 1302 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.883 (1H, t, J = 7.8, J = 7.5, NH), 7.759–8.203 (14H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm. 116.47, 118.49, 121.01, 126.19, 127.25, 129.02, 130.04, 134.49, 136.34, 137.02, 142.16, 143.9, 147.84, 149.34, HRMS (ESI): 344.612 obs, 345.35 calc.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1556 (C–Cl), 1414 (–N
C–), 1556 (C–Cl), 1414 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3): δH ppm 7.193 (1H, s, NH) 7.612–7.639 (13H, m, Ar–H), 13C NMR (CDCl3,75 MHz): 77.41, 115.43, 118.95, 123.20, 123.62, 128.41, 128.98, 129.47, 129.70, 130.33, 130.56, 130.78, 131.07, 133.49, 145.12, HRMS (ESI): 369.0617 (M+) calc. 369.25.
N–), 1H-NMR (300 MHz, CDCl3): δH ppm 7.193 (1H, s, NH) 7.612–7.639 (13H, m, Ar–H), 13C NMR (CDCl3,75 MHz): 77.41, 115.43, 118.95, 123.20, 123.62, 128.41, 128.98, 129.47, 129.70, 130.33, 130.56, 130.78, 131.07, 133.49, 145.12, HRMS (ESI): 369.0617 (M+) calc. 369.25.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3): δH ppm 6.619 (1H, s, N–H), 7.648–7.681 (14H, m, Ar–H), 2.524 (3H,–COCH3), 13C NMR (CDCl3,75 MHz): 26.03, 112.59, 113.60, 119.87, 126.05, 127.50, 128.27, 128.50, 129.20, 130.75, 135.25, 137.18, 144.60, 151.19, 196.73 HRMS (ESI): 342.32 (M+) calc. 342.39.
N–), 1H-NMR (300 MHz, CDCl3): δH ppm 6.619 (1H, s, N–H), 7.648–7.681 (14H, m, Ar–H), 2.524 (3H,–COCH3), 13C NMR (CDCl3,75 MHz): 26.03, 112.59, 113.60, 119.87, 126.05, 127.50, 128.27, 128.50, 129.20, 130.75, 135.25, 137.18, 144.60, 151.19, 196.73 HRMS (ESI): 342.32 (M+) calc. 342.39.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1556 (–C
C–), 1556 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1412 (–N
N–), 1412 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3) δH ppm 2.258 (3H, s), 6.625 (1H, d, J = 8.4, NH), 7.004–8.24 (14H, m, Ar–H), 13C NMR (75 MHz, CDCl3) δ ppm 146.81, 143.55, 141.76, 133.73, 129.92, 129.72, 129.38, 129.22, 127.88, 126.12, 124.03, 123.94, 123.56, 121.1, 115.31, 113, 20.42. HRMS (ESI): 344.612 calc. 243.
N–), 1H-NMR (300 MHz, CDCl3) δH ppm 2.258 (3H, s), 6.625 (1H, d, J = 8.4, NH), 7.004–8.24 (14H, m, Ar–H), 13C NMR (75 MHz, CDCl3) δ ppm 146.81, 143.55, 141.76, 133.73, 129.92, 129.72, 129.38, 129.22, 127.88, 126.12, 124.03, 123.94, 123.56, 121.1, 115.31, 113, 20.42. HRMS (ESI): 344.612 calc. 243.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1414 (–N
C–), 1414 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3): δH ppm 1.798 (3H, s, CH3), 6.622 (1H, s, N–H) 6.650–7.627 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 20.13, 117.29, 118.59, 122.27, 124.65, 126.80, 127.60, 28.81, 130.05, 132.11, 137.25, 138.97, 144.62, 151.12, HRMS (ESI): 349.872 (obs) 348.5 (calc.).
N–), 1H-NMR (300 MHz, CDCl3): δH ppm 1.798 (3H, s, CH3), 6.622 (1H, s, N–H) 6.650–7.627 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 20.13, 117.29, 118.59, 122.27, 124.65, 126.80, 127.60, 28.81, 130.05, 132.11, 137.25, 138.97, 144.62, 151.12, HRMS (ESI): 349.872 (obs) 348.5 (calc.).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1419 (–N
N), 1419 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3): δH ppm 3.933 (3H, d, J = 13.4), 6.559 (1H, s, N–H), 6.587–8.125 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 50.02, 112.72, 120.28, 126.43, 126.80, 128.28, 128.99, 129.25, 129.57, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79, HRMS (ESI): 349.321 (M+) obs, 348.5 calc.
N–), 1H-NMR (300 MHz, CDCl3): δH ppm 3.933 (3H, d, J = 13.4), 6.559 (1H, s, N–H), 6.587–8.125 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 50.02, 112.72, 120.28, 126.43, 126.80, 128.28, 128.99, 129.25, 129.57, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79, HRMS (ESI): 349.321 (M+) obs, 348.5 calc.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1415 (–N
N str), 1415 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1H-NMR (300 MHz, CDCl3): δH ppm 2.956 (3H, s, OCH3), 6.925 (s, N–H), 6.950–7.489 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3) δ ppm 50.02, 112.72, 120.28, 126.43, 126.80, 128.28, 128.99, 129.57, 129.25, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79. HRMS (ESI): 376.1 calc. 376.84.
N–), 1H-NMR (300 MHz, CDCl3): δH ppm 2.956 (3H, s, OCH3), 6.925 (s, N–H), 6.950–7.489 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3) δ ppm 50.02, 112.72, 120.28, 126.43, 126.80, 128.28, 128.99, 129.57, 129.25, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79. HRMS (ESI): 376.1 calc. 376.84.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1540 (–NO2), 1410 (–N
C–), 1540 (–NO2), 1410 (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1301 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 2.568 (3H, s, –CH3), 6.625 (1H, s, N–H), 6.653–8.240 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 20.42, 77.43, 113.00, 115.31, 121.10, 123.56, 123.94, 124.03, 126.12, 127.88, 129.22, 129.92, 133.73, 141.76, 143.55, 146.81. HRMS (ESI): 360.12 (M+) obs, 359 (calc.).
N–), 1301 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 2.568 (3H, s, –CH3), 6.625 (1H, s, N–H), 6.653–8.240 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 20.42, 77.43, 113.00, 115.31, 121.10, 123.56, 123.94, 124.03, 126.12, 127.88, 129.22, 129.92, 133.73, 141.76, 143.55, 146.81. HRMS (ESI): 360.12 (M+) obs, 359 (calc.).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1548 (–NO2), 1418(–N
C–), 1548 (–NO2), 1418(–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1299 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.612 (1H, s, N–H), 6.64–8.174 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 77.91, 113.66, 117.86, 121.82, 127.76, 128.43, 129.42, 130.00, 130.78, 136.08, 138.21, 147.85, 151.05, 152.08. HRMS (ESI): 391.21 (M+ obs) 390 (calc.).
N–), 1299 (–NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.612 (1H, s, N–H), 6.64–8.174 (13H, m, Ar–H), 13CNMR (75 MHz, CDCl3): δ ppm 77.91, 113.66, 117.86, 121.82, 127.76, 128.43, 129.42, 130.00, 130.78, 136.08, 138.21, 147.85, 151.05, 152.08. HRMS (ESI): 391.21 (M+ obs) 390 (calc.).Conclusion
In all previously reported synthetic protocols, either the aldehyde phenylhydrazones or the diazonium ions or both were prepared separately and brought into reaction in a suitable medium. In this work the possibility of a one pot procedure in the absence of any organic solvent or added coupling agents was explored. The solid phase synthesis suffered from the drawback of being a multistep synthetic procedure and moderate yield obtained due to incomplete extraction and therefore cannot be termed as conforming to the provisions of green chemistry whereas the use of ionic liquid as the catalyst resulted in a one pot environmentally benign synthesis with high yield and in a very short reaction time. Further the reaction, catalyzed by ionic liquid as reported herein, need not be carried out at low temperatures which make the procedure suitable for industrial application for cost effective, environmentally benign and large scale production of formazans dyes.Acknowledgements
The authors are thankful to the HOD, Department of Chemistry, Gauhati University, for providing laboratory facilities and Prof. A. K. Saikia, IIT, Guwahati for instrumental facilities.Notes and references
- H. Pechmann, Berichte, 1892, 25, 3175 CrossRef PubMed.
- E. Bamberger and E. W. Wheelwright, Berichte, 1892, 25, 3201 CrossRef PubMed.
- S. Sanli, N. Sanli and G. Alsancak, J. Braz. Chem. Soc., 2009, 20, 939 CAS.
- J. L. Beltran, N. Sanli, G. Fonrodona, D. Barren, G. Özkan and J. Barbosa, Anal. Chim. Acta, 2003, 484, 253 CrossRef CAS.
- H. Senöz, J. Biol. Chem., 2012, 40, 293 Search PubMed.
- P. M. Debnath and G. Shearer, Anal. Biochem., 1997, 250, 253 CrossRef PubMed.
- B. N. Barsoum, S. K. Khella, A. H. M. Elwaby, A. A. Abbas and Y. A. Ibrahim, Talanta, 1998, 47, 1215 CrossRef CAS.
- J. H. P. Van Aardt, W. Tadros, H. J. Cottrell, D. L. Pain, R. Slack, J. C. D. Brand, G. J. Minkoff, M. F. R. Mulcahy, I. C. Watt, E. R. Ward, T. M. Coulson, J. W. Hawkins, K. Balenovic, D. Dvornik, J. P. Blanchard, H. L. Goering, E. S. Lane and C. Williams, J. Chem. Soc., 1954, 2967 Search PubMed.
- V. S. Misra, S. Dhar and B. L. Chowdhary, Pharmazie, 1978, 33, 790 CAS.
- D. D. Mukherjee, S. K. Shukla and B. L. Chowdhary, Arch. Pharma., 1981, 314, 991 CrossRef PubMed.
- R. M. Desai and J. M. Desai, Indian J. Heterocycl. Chem., 1999, 8, 329 Search PubMed.
- A. B. Samel and N. R. Pai, J. Chem. Pharm. Res., 2010, 2, 60 CAS.
- R. Kalsi, K. Pande, T. N. Bhalla, S. S. Parmar and J. P. Brathwal, Pharmacology, 1988, 37, 218 CrossRef CAS PubMed.
- K. G. Desai and K. R. Desai, J. Heterocycl. Chem., 2006, 43, 1083 CrossRef CAS PubMed.
- A. W. Nineham, Chem. Rev., 1955, 55, 355 CrossRef CAS.
- J. N. Ashley, B. M. Davis, A. W. Nineham and R. Slack, J. Chem. Soc., 1953, 3881 RSC.
- S. H. Korzeniowski and G. W. Gokel, Tetrahedron Lett., 1977, 1637 CrossRef CAS.
- A. R. Butler and P. T. Shepard, J. Chem. Res., Synop., 1978, 339 CAS.
- H. Iwamoto, M. Yoshimura, T. Sonoda and H. Kobayashi, Bull. Chem. Soc. Jpn., 1985, 56, 796 CrossRef.
- Y. Hashida, K. Kubota and S. Sekiguchi, Bull. Chem. Soc. Jpn., 1988, 61, 905 CrossRef CAS.
- A. R. Katritzky, S. A. Belyakov, D. Cheng and H. Dupont Durst, Synthesis, 1995, 577 CrossRef CAS PubMed.
- H. Tezcan and E. Uzluk, Dyes Pigm., 2007, 75, 633 CrossRef CAS PubMed.
- H. Tezcan and G. Ekmekci, Acta Chim. Slov., 2010, 57, 189 CAS.
- H. Tezcan, S. Can and R. Tezcan, Dyes Pigm., 2002, 52, 121 CrossRef CAS.
- H. Tezcan and N. Ozkan, Dyes Pigm., 2003, 56, 159 CrossRef CAS.
- H. Tezcan and N. Ozbek, Commun. Fac. Sci. Univ. Ankara, Ser. B: Chem. Chem. Eng., 2005, 51, 13 CAS.
- A. Bamoniri, B. B. F. Mirjalili and N. Moshtad Arani, J. Mol. Catal. A: Chem., 2014, 393, 272 CrossRef CAS PubMed.
- J. Dupont, R. F. De Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed.
- J. S. Wilkes, Green Chem., 2002, 4, 73 RSC.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2002, 39, 3772 CrossRef.
- W. Welton, Chem. Rev., 1999, 99, 2971 CrossRef PubMed.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- R. T. Carlin and J. S. Wilkes, in Advances in non aqueous Chemistry, ed. G. Mamantov and A. Popov, VCH, New York, 1994 Search PubMed.
- Y. Chauvin and H. Olivier, J. Mol. Catal. A: Chem., 1999, 146, 285 CrossRef.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC.
- F. Bigi, R. Maggi and G. Sartori, Green Chem., 2000, 2, 140 RSC.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 CrossRef CAS.
- A. K. Burrel, R. E. Del Sesto, S. N. Baker, T. M. McCleskey and G. A. Baker, Green Chem., 2007, 9, 449 RSC.
- Y. U. Paulechka, G. J. Kabo, A. V. Blokhin, O. A. Vydror, J. W. Magee and M. Frenkel, J. Chem. Eng. Data, 2003, 48, 457 CrossRef CAS.
- G. J. Kabo, A. V. Blokhin, Y. U. Paulechka, A. G. Kabo and J. W. Magee, J. Chem. Eng. Data, 2004, 49, 453 CrossRef CAS.
- J. Weng, C. Wang, H. Li and Y. Wang, Green Chem., 2006, 8, 96 RSC.
- R. S. Varma and V. V. Namboodri, Pure Appl. Chem., 2001, 73, 1309 CAS.
- N. Gathergood, M. T. Barcia and P. J. Scammells, Green Chem., 2004, 6, 166 RSC.
- P. J. Das and S. Khound, Tetrahedron, 1994, 50(1994), 9107 CrossRef CAS.
- P. J. Das and S. Khound, Tetrahedron, 1997, 53, 9749 CrossRef.
- P. J. Das and S. Khound, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1998, 37, 155 Search PubMed.
- P. J. Das and J. Das, RSC Adv., 2015, 5, 11745 RSC.
- H. Senoz, Hacettepe J. Biol. Chem., 2012, 40, 293 Search PubMed.
- Vogel's Textbook of Practical Organic Chemistry, ed. B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, Pearson Education, 5th edn, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra, HRMS data of all new compounds synthesized, their methods of preparations graphics of the reaction sequence. See DOI: 10.1039/c5ra06363a |
| This journal is © The Royal Society of Chemistry 2015 |