Cellulosic poly(ionic liquid)s: synthesis, characterization and application in the cycloaddition of CO2 to epoxides†
Qin Chenab,
Chang Pengb,
Haibo Xie*b,
Zongbao kent Zhao*b and
Ming Baoa
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116023, PR China
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, CAS, Dalian 116023, PR China. E-mail: hbxie@dicp.ac.cn; zhaozb@dicp.ac.cn
First published on 8th May 2015
Abstract
Cellulosic poly(ionic liquid)s (PILs) were prepared via nucleophilic substitution of chlorinated cellulose by 1-methyl-imidazole and their structure and thermal properties have been fully characterized; the cellulosic PILs were found to be green, efficient and recyclable catalysts for the cycloaddition of CO2 to epoxides under solvent-free conditions.
Ionic liquids (ILs) are molten salts at relatively low temperatures and exhibit a series of unique properties compared with molecular solvents and materials, which make them interesting as new solvents, catalysts and functional materials.1–3 The introduction of an IL moiety into polymers results in a new family of polymeric materials, poly(ionic liquid)s (PILs), with particular properties and interesting applications.4–7 For example, considering the catalytic performance of an ionic liquid moiety, PILs could be used as efficient heterogeneous catalysts with enhanced recyclability, such as cyclic addiction of epoxides with CO2 to prepare cyclic carbonates.8–10
Up to now great efforts have been devoted to synthesize PILs and two distinctive strategies have been developed: (1) polymerization of polymerizable ILs monomers, (2) post-modification of existing polymers.5 Generally, the polymerizable ILs monomers containing (meth)acryloyl,11,12 styrenic13 or vinyl14 groups can be employed to synthesize PILs via traditional polymerization technologies. PILs with special polymeric backbones can be achieved by post-modification of either of synthetic polymers or biopolymers.15–17 Usually, a chloromethyl is introduced onto the synthetic polymers firstly, followed by an alkylation of Lewis organic bases and anion exchange reaction to obtain the designable PILs.18,19
Compared with those synthetic polymers, the use of biopolymers as backbone of PILs is anticipated to provide advantages of natural abundance, low cost, nontoxicity, biocompatibility and biodegradability.20 The preparation of biopolymers-based PILs is mainly depended on the chemical reactions of its hydroxyl groups. For example, the cellulose-based PILs (quaternized cellulose) have been synthesized via etherification reaction and widely used in various fields for a long time, such as environmental protection, water treatment, textile dyeing industry17,21 and gene transfection.22 Among the etherification reagents, the 3-chloro-2-hydroxypropyl trimethylammonium chloride (CHTAC) has been the most preferred one.22–24 The etherification reaction is usually carried out under aqueous alkaline conditions, and excess amount of sodium hydroxide is usually required to achieve satisfactory modification efficiency. Additionally, the etherification protocol also suffers from drawbacks such as side reaction, low modification efficiency, and low structural diversity.25,26 Another choice of biopolymer as a backbone of PILs is chitosan due to its active functional groups, including hydroxyl groups and amino groups. For example, chitosan-based PILs have been prepared via a direct alkylation of amino group, which were successfully used in green catalysis.27–29 He L. N. et al. prepared chitosan supported PILs via the reaction between chitosan and CHTAC.30 Park D. W. proposed another method in which the quaternization is occurred on the primary amine on chitosan rather than anchoring a prebuilt quaternized group onto chitosan.31,32 Dupont et al. demonstrated that imidazolium-based ILs were great candidates for the formation and stabilization of metal nanoparticles.3,33,34 Thereby it is still incentive to develop more facile route to prepare cellulosic PILs, especially with the imidazolium group.
Inspired by the commonly used protocol for the preparation of imidazolium ILs (Scheme 1), herein, we report a facile protocol for the preparation of cellulosic PILs, cellulose anchored 1-methyl-imidazolium chloride ([Cellmim][Cl]), via alkylation reaction between chlorinated cellulose and 1-methyl-imidazole. The as-prepared cellulosic PILs were applied in the cycloaddition of CO2 to epoxides under solvent-free conditions as a proof and concept application.
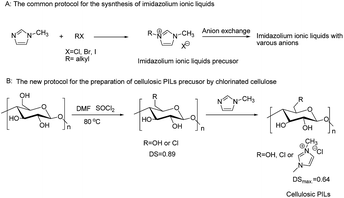 | ||
| Scheme 1 Facile preparation of cellulosic PILs by using chlorinated cellulose and 1-methyl-imidazole. | ||
Cellulose was chemically modified with SOCl2 to obtain chlorinated cellulose (6-chloro-6-deoxycellulose, CDC) with a DS of 0.89 preferentially on C-6 position, which was in accordance with previous publications.35,36 Compared with other classes of ionic liquids the imidazolium-based ionic liquids are one of the most important families of ionic liquids due to their outstanding properties.1 We started the study with 1-methyl-imidazole to prepare the [Cellmim][Cl] both without solvent and in DMSO or 1-allkyl-3-methyl-imidazolium chloride (AmimCl). The degree of substitution (DS) of the [Cellmim][Cl] was calculated according to elemental analysis results of nitrogen content and the reaction efficiency (RE) was calculated from the DS of CDC and the [Cellmim][Cl]. It was found that the alkylation reaction could proceed smoothly at 100 °C, and [Cellmim][Cl] with a DS of 0.56 was obtained in 24 h when excess 1-methyl-imidazole was used as a reagent and solvent (Table 1, entry 3). Further prolonging the reaction time to 48 h, a maximal DS of 0.64 was achieved (Table 1, entry 4). Correspondingly, the RE of the chloromethyl on cellulose was 71.9%. Although the DS increased significantly, the yield did not change notably, which suggested that degradation of the chlorinated cellulose occurred during the reaction. The reaction temperature has an great affect on the reaction, which was evidenced by the DS significantly decreased to 0.37 and 0.14 when the temperature was decreased to 90 °C and 80 °C in 24 h, respectively (Table 1, entries 1 and 2). It was also found that the reaction started in heterogeneous conditions and then became highly swollen gel-like state with high viscosity, which confirmed the successful alkylation reaction. In order to decrease the viscosity of the reaction mixture, DMSO and AmimCl were used as solvents for the alkylation reaction under identical conditions. It was interesting to find that the reaction mixture was also heterogeneous at the beginning in the case of DMSO, but became homogeneous in shorter time. Although higher yields were obtained in this case, the [Cellmim][Cl] only have moderate DS of 0.35 and 0.42 at 90 °C and 100 °C, respectively (Table 1, entries 5 and 6), which were lower than those obtained under neat conditions (Table 1, entries 2 and 3). The results suggest that the use of DMSO as solvent cannot promote the alkylation reaction, but can suppress the degradation of CDC. In the case of AmimCl, the reaction was performed under homogeneous condition as the ionic liquids were good solvents to CDC; surprisingly, the product with lower DS of 0.3 was obtained, which were similar to the amination of cellulose.35 It seems that the stronger ionic strength of ionic liquids suppressed the alkylation reaction although a homogeneous state was sustained all the time.
| Entry | Solvent | T °C | t h | N% | DS | Yield% | RE% |
|---|---|---|---|---|---|---|---|
| a 0.5 mL 1-methyl imidazole, 0.5 g CDC.b 0.5 mL DMSO.c 12.5 g AmimCl. | |||||||
| 1 | None | 80 | 24 | 2.049 | 0.14 | 79.3 | 15.7 |
| 2 | None | 90 | 24 | 5.003 | 0.37 | 89.6 | 41.6 |
| 3 | None | 100 | 24 | 6.976 | 0.56 | 83.5 | 62.9 |
| 4 | None | 100 | 48 | 7.752 | 0.64 | 84.5 | 71.9 |
| 5 | DMSOb | 90 | 72 | 4.751 | 0.35 | 97.5 | 39.3 |
| 6 | DMSOb | 100 | 24 | 5.517 | 0.42 | 89.2 | 47.2 |
| 7 | AmimClc | 90 | 24 | 4.176 | 0.30 | 84.4 | 33.7 |
The solubility of [Cellmim][Cl] with different DS values in conventional solvents was also studied (Table S1†). Their solubility mostly depends on the DS values and the samples with DS > 0.37 are soluble in water; the sample with a DS of 0.14 only can be swollen by water, but is soluble in DMSO. It is also interesting to find that the samples with DS < 0.4 are soluble in DMSO; while the samples with DS > 0.4 can be only swollen by DMSO, thus achieving a gel-like solution with enhanced viscosity. It is worthy to mention that the good solubility of the [Cellmim][Cl] in water and DMSO will provide great opportunities to prepare functional composites materials with other functional components through solution processing and homogeneous catalysis.
The successful preparation of chlorinated cellulose and [Cellmim][Cl] was confirmed by comparative CP/MAS 13C NMR spectra as shown in Fig. 1. The spectrum of pure cellulose show typical peaks for its six carbons in the monomer unit. The downfield peak at 104.7 ppm is assigned to C-1 since C-1 is bonded to two oxygen atoms. The peak at 88.6 ppm is assigned to C-4 which is connected to only one oxygen and is responsible for β-1,4-glucoside bonds. The chemical shift at 74.7 and 72.0 ppm are classified to C-2, C-3 and C-5 because they are all secondary carbons attached to –CH or –OH group. The peak of C-6 appears at 65.0 ppm, which is a primary carbon connected to –OH and –CH2 groups. The partial substitution of the –OH by chloride at C-6 resulted in a significant change of the chemical shift of C-6 from 65.0 to 44.8 ppm. The substitution only resulted in slight changes of the chemical shifts of C-1 from 104.7 to 103.7 ppm, of C-4 from 88.6 to 83.9 ppm, while no notable affect on the chemical shifts of C-2, C-3 and C-5 was observed. These results confirm the successful preparation of chlorinated cellulose in this study.37
With the covalent bonding the 1-methyl-imidazolium chloride moiety onto cellulose, new signals at 35.9 (C-10), 123.2 (C-8, 9), and 137.6 (C-7) appeared, assigning to the imidazolium ionic liquids moiety, and the reaction resulted in a change in the chemical shift of C-6 from 44.8 to 50.3 ppm, and no obvious affect on the chemical shifts of C1–C5 carbons. A signal of chloromethyl carbon were still detected in the spectra of [Cellmim][Cl]DS=0.64, indicating the presence of chloromethyl residual, which was in accordance with the fact that the DS of ionic liquids moiety is lower than the DS of chlorinated cellulose. We further applied the multi-peaks analysis using Lorentzian method in Origin software to separate the overlapped peaks (Fig. 1c, C6, C6′) and calculate the corresponding areas. By compare the areas of the separated peaks, the DS value of methyl imidazolium chloride and chloride is estimated to be 0.64 and 0.36, respectively. The results are in good accordance with the elemental analysis. The structure of [Cellmim][Cl]DS=0.64 was further confirmed by HSQC in D2O (Fig. S1†), and the assignments was summarized in Table S2.† The results are in accordance with that of solid state 13C NMR.
The successful chemical modification of cellulose was also confirmed by FTIR analysis (Fig. 2). In comparison with the spectrum of raw cellulose, two new bands at 752 and 723 cm−1 appeared in the spectrum of the chlorinated cellulose, which were corresponded to stretching vibration of the C–Cl bond. In association with the appearance of the new bands, the bands from 1500 to 1200 cm−1 decreased owing to the substitution of the hydroxyl group on C-6.37 In contrast, new characteristic bands centered at 1577, 1114, and 1065 cm−1 corresponded to the imidazolium moiety appeared in the spectrum of [Cellmim][Cl]DS=0.64,38 and the intensity of the band centered at 3400 cm−1 increased due to the hydrophilic property of the 1-methyl-imidazolium chloride moiety.
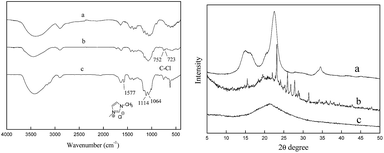 | ||
| Fig. 2 FTIR and X-ray diffraction patterns of cellulose (a) CDC (b) DS = 0.89, and [Cellmim][Cl]DS=0.64 (c). | ||
The changes of the molecular-level aggregation structure of the polymer arose by the chemical modifications could be followed by X-ray diffraction technology. The X-ray diffraction pattern of raw cellulose shows three distinctive peaks corresponding to 101, 002 and 040 planes which are characteristic of microcrystalline cellulose (Fig. 2).35 In contract, the chlorinated cellulose exhibits new crystal peaks but with lower intensity, different from those observed for raw cellulose, which indicate that new assembly is formed via the bonded chlorine atoms and the remaining hydroxyl groups.35,37 The decreased crystallinity of chlorinated cellulose will be very interesting mainly when subsequent modification are intended, such as anchoring a 1-methyl-imidazolium moiety to the backbone. For the [Cellmim][Cl]DS=0.64, as shown in Fig. 2, a drastic decrease in crystallinity was observed in comparison with the raw cellulose and CDC. These data suggest that the cellulosic PILs have an amorphous structure, and the newly anchored groups do not establish an ordered assembly.
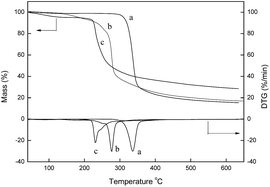
Further thermogravimetric analysis of cellulose, CDC and [Cellmim][Cl]DS=0.64 were shown in Fig. 3. Both of cellulose derivatives exhibited inferior thermal stability to the raw cellulose, as illustrated in thermogravimetric curves. Cellulose can remain stable up to 300 °C and shows only one decomposing process from 300 to 380 °C with a mass loss of 92% owing to the pyrolytic degradation of the carbon skeleton. The onset degradation temperature of CDC and [Cellmim][Cl]DS=0.64 is 266 and 220 °C, respectively, and their mass loss even after 600 °C is estimated to be 83% and 71%, respectively. The lower degradation temperature was attributed to the destroyed crystalline structure and the different chemical structures. In addition, both CDC and [Cellmim][Cl]DS=0.64 gave two stage of decomposition behavior. For CDC the first mass loss between 150 and 250 °C is caused by the cleavage of C–Cl bond and the condensation of hydroxyl groups on C-2 and C-3. The second stage from 250 °C is corresponding to the depolymerization of cellulose polymeric chain.39,40 The degradation behavior of [Cellmim][Cl]DS=0.64 is similar to that of CDC, with the initial mass loss from 220 to 280 °C which can be interpreted as losing of the immobilized imidazulium group on cellulose backbone together with the condensation of hydroxyl groups on C-2 and C-3. The second step is also correlated with the loss of cellulose fiber.
The thermal properties of the as-prepared cellulose derivatives were further investigated by differential scanning calorimetry. The CDC exhibits typical crystal structure with melting peak at 119.27 °C and the corresponding enthalpy is 2.716 J g−1 from the first heating cycle (Fig. 4A). Additionally, the crystallization temperature (Tc) appeared at −19.64 °C with ΔHc value of −0.193 J g−1. The glass transfer temperature was observed from the second heating cycle at −40.29 °C. On the other hand, the [Cellmim][Cl]DS=0.64 was found to be amorphous but the obvious grass transition stage was not observed in neither first nor second heating curve (Fig. 4B). The Tc of [Cellmim][Cl]DS=0.64 appeared at −11.92 °C with ΔHc value of −0.469 J g−1 from the first heating cycle.
 | ||
| Fig. 4 DSC curve of CDC (DS = 0.89 (A)) and [Cellmim][Cl]DS=0.64 (B). (a) First heating cycle, (b) second heating cycle. | ||
The design and preparation of PILs have been demonstrated to broaden the properties and applications of ionic liquids, which have been reviewed in recent comprehensive reviews.4,5,7,41 With the new cellulosic PILs ([Cellmim][Cl]) in hand, it is reasonable to conjecture that its physical and chemical properties can be tuned via the freedom combination of this cellulosic poly(cation) and anions due to the designable nature of the anchored 1-methyl-imidazolium chloride moiety.42–44 The direct use of PILs as catalysts or as the supports for catalytic active species in various catalytic reactions has been widely investigated.7–10 Among the various reactions catalyzed by PILs, one of the most concerned topics is the synthesis of cyclic carbonates via its addition reaction of CO2 and epoxides.8–10,27,42 Therefore, the reaction was also selected as a proof and concept application of the [Cellmim][Cl]DS=0.64 to demonstrate its potential. To our delight, the catalytic activity varied with the halide anion (Table 2), and iodide exhibited the highest yield while chloride showed the lowest yield in the cycloaddition of CO2 to propylene oxide. The difference may be ascribed to the different nucleophilicity and leaving ability of the halide anions, which is in accordance with the trend in previous studies.45 The catalytic system could also be extended to the reactions of different epoxides with CO2, and good isolated yields of cyclic-carbonates were achieved.
| Entry | Catalysts | Epoxide | Product | bYield% |
|---|---|---|---|---|
| a Reaction conditions: epoxides 20 mmol, 5 mol% cellulosic PILs, CO2 pressure 2 MPa, 120 °C 6 h.b Isolated yield.c 150 °C. | ||||
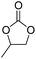
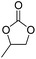
| 1 | [Cellmim][Cl]DS=0.64 | 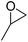 |
 |
44.2 |
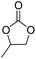
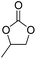
| 2 | [Cellmim][Br]DS=0.64 | 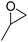 |
 |
68.7 |
| 3 | [Cellmim][I]DS=0.64 | 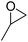 |
 |
80.4 |
| 4c | [Cellmim][I]DS=0.64 | 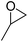 |
 |
95.1 |
| 5 | [Cellmim][Br]DS=0.64 | 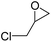 |
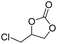 |
87.4 |
| 6 | [Cellmim][I]DS=0.64 | 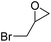 |
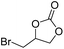 |
88.7 |
| 7 | [Cellmim][I]DS=0.64 | 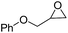 |
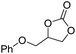 |
95.3 |
| 8 | [Cellmim][I]DS=0.64 | 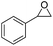 |
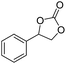 |
91.7 |
The reusability of the [Cellmim][I]DS=0.64 catalyst was studied in the cycloaddition of glycidyl phenyl ether and CO2. The catalyst could be separated by washing with ethyl acetate, filtered and then dried under vacuum. The recovered catalyst was directly employed for the cycloaddition under identical conditions. The catalyst remained active up to 5 runs without remarkable decrease in yield demonstrating high reusability of the catalyst (Fig. 5). Further analysis of the recovered catalyst by FTIR, 1H NMR and TGA were performed towards a better understanding of the potential structural changes on the catalyst during the reusability study, and the comparative results were shown in Fig. S2 to S4.† The structural information from the 1H NMR and FTIR spectra of the recovered catalyst demonstrated that the chemical bonded imidazolium moiety was stable during the reaction, and the structure remained unchanged after 5 cycles, which determined its stable catalytic activity during the reusability study. The TGA result (Fig. S4†) showed that the onset temperature of recovered catalyst remained unchanged but the residual weight at 650 °C decreased from 38.9% to 31.0%. The possible reason for this is still unclear at the early stage of the study.
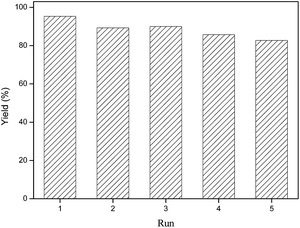 | ||
| Fig. 5 Reusability of [Cellmim][I]DS=0.64. Reaction conditions: glycidyl phenyl ether (20 mmol), [Cellmim][I]DS=0.64 (5 mmol%), 120 °C, 2 MPa, 6 h. | ||
In summary, the chemical modification of cellulose with SOCl2 produced CDC with a DS of 0.89, which was successfully used as alkylation reagent for 1-methyl-imidazole to synthesize cellulosic PILs with tunable substitution degrees. The structural and thermal properties of the cellulosic PILs were studied by various technologies, and they were ready for the preparation of novel cellulosic functional materials. As a proof and concept application, the as-prepared cellulosic PILs were used as green, efficient and recyclable catalysts for the cycloaddition of CO2 to epoxides under solvent-free conditions. Further work in this area will focus on the design and application the derivative functional materials by taking the advantages of cellulose and ionic liquids moieties.
Acknowledgements
This work was supported by Natural Science Foundation of China (no. 21002101).Notes and references
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- T. Torimoto, T. Tsuda, K.-i. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196–1221 CrossRef CAS PubMed.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009–1036 CrossRef CAS PubMed.
- J. Yuan and M. Antonietti, Polymer, 2011, 52, 1469–1482 CrossRef CAS PubMed.
- B. Xin and J. Hao, Chem. Soc. Rev., 2014, 43, 7171–7187 RSC.
- P. Zhang, T. Wu and B. Han, Adv. Mater., 2014, 26, 6810–6827 CrossRef CAS PubMed.
- S. Ghazali-Esfahani, H. Song, E. Paunescu, F. D. Bobbink, H. Liu, Z. Fei, G. Laurenczy, M. Bagherzadeh, N. Yan and P. J. Dyson, Green Chem., 2013, 15, 1584–1589 RSC.
- T.-Y. Shi, J.-Q. Wang, J. Sun, M.-H. Wang, W.-G. Cheng and S.-J. Zhang, RSC Adv., 2013, 3, 3726–3732 RSC.
- Y. Xie, Z. Zhang, T. Jiang, J. He, B. Han, T. Wu and K. Ding, Angew. Chem., Int. Ed., 2007, 46, 7255–7258 CrossRef CAS PubMed.
- J. Huang, C.-a. Tao, Q. An, W. Zhang, Y. Wu, X. Li, D. Shen and G. Li, Chem. Commun., 2010, 46, 967–969 RSC.
- F. Yan and J. Texter, Chem. Commun., 2006, 2696–2698 RSC.
- J. E. Bara, C. J. Gabriel, E. S. Hatakeyama, T. K. Carlisle, S. Lessmann, R. D. Noble and D. L. Gin, J. Membr. Sci., 2008, 321, 3–7 CrossRef CAS PubMed.
- R. Marcilla, J. Alberto Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 208–212 CrossRef CAS PubMed.
- W. Zhang, H. Wang, J. Han and Z. Song, Appl. Surf. Sci., 2012, 258, 6158–6168 CrossRef CAS PubMed.
- B. Motos-Perez, J. Roeser, A. Thomas and P. Hesemann, Appl. Organomet. Chem., 2013, 27, 290–299 CrossRef CAS PubMed.
- M. M. Kamel, M. M. El Zawahry, N. S. E. Ahmed and F. Abdelghaffar, Ind. Crops Prod., 2011, 34, 1410–1417 CrossRef CAS PubMed.
- J. Wang, S. Li and S. Zhang, Macromolecules, 2010, 43, 3890–3896 CrossRef CAS.
- B. Lin, H. Dong, Y. Li, Z. Si, F. Gu and F. Yan, Chem. Mater., 2013, 25, 1858–1867 CrossRef CAS.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 52, 1096–1108 CrossRef CAS PubMed.
- S. Acharya, N. Abidi, R. Rajbhandari and F. Meulewaeter, Cellulose, 2014, 21, 4693–4706 CrossRef CAS PubMed.
- Y. Song, Y. Sun, X. Zhang, J. Zhou and L. Zhang, Biomacromolecules, 2008, 9, 2259–2264 CrossRef CAS PubMed.
- T. S. De Vries, D. R. Davies, M. C. Miller and W. A. Cynecki, Ind. Eng. Chem. Res., 2014, 53, 9686–9694 CrossRef CAS.
- M. Hashem, P. Hauser and B. Smith, Text. Res. J., 2003, 73, 1017–1023 CrossRef CAS PubMed.
- H. S. Seong and S. W. Ko, J. Soc. Dyers Colour., 1998, 114, 124–129 CrossRef CAS PubMed.
- K. R. Roshan, T. Jose, A. C. Kathalikkattil, D. W. Kim, B. Kim and D. W. Park, Appl. Catal., A, 2013, 467, 17–25 CrossRef CAS PubMed.
- J. Baudoux, K. Perrigaud, P.-J. Madec, A.-C. Gaumont and I. Dez, Green Chem., 2007, 9, 1346–1351 RSC.
- N. Clousier, A. Filippi, E. Borré, E. Guibal, C. Crévisy, F. Caijo, M. Mauduit, I. Dez and A.-C. Gaumont, ChemSusChem, 2014, 7, 1040–1045 CrossRef CAS PubMed.
- J. Sun, J. Wang, W. Cheng, J. Zhang, X. Li, S. Zhang and Y. She, Green Chem., 2012, 14, 654–660 RSC.
- Y. Zhao, J.-S. Tian, X.-H. Qi, Z.-N. Han, Y.-Y. Zhuang and L.-N. He, J. Mol. Catal. A: Chem., 2007, 271, 284–289 CrossRef CAS PubMed.
- J. Tharun, Y. Hwang, R. Roshan, S. Ahn, A. C. Kathalikkattil and D.-W. Park, Catal. Sci. Technol., 2012, 2, 1674–1680 CAS.
- J. Tharun, D. W. Kim, R. Roshan, Y. Hwang and D. W. Park, Catal. Commun., 2013, 31, 62–65 CrossRef CAS PubMed.
- J. Dupont, G. S. Fonseca, A. P. Umpierre, P. F. P. Fichtner and S. R. Teixeira, J. Am. Chem. Soc., 2002, 124, 4228–4229 CrossRef CAS PubMed.
- P. Migowski, G. Machado, S. R. Texeira, M. C. M. Alves, J. Morais, A. Traverse and J. Dupont, Phys. Chem. Chem. Phys., 2007, 9, 4814–4821 RSC.
- E. C. da Silva Filho, S. A. A. Santana, J. C. P. Melo, F. J. V. E. Oliveira and C. Airoldi, J. Therm. Anal. Calorim., 2010, 100, 315–321 CrossRef CAS PubMed.
- T. Tashiro and Y. Shimura, J. Appl. Polym. Sci., 1982, 27, 747–756 CrossRef CAS PubMed.
- E. C. Silva Filho, L. C. B. Lima, F. C. Silva, K. S. Sousa, M. G. Fonseca and S. A. A. Santana, Carbohydr. Polym., 2013, 92, 1203–1210 CrossRef CAS PubMed.
- J. Sun, W. Cheng, W. Fan, Y. Wang, Z. Meng and S. Zhang, Catal. Today, 2009, 148, 361–367 CrossRef CAS PubMed.
- N. Kasuya, T. Suzuki and A. Sawatari, J. Wood Sci., 1999, 45, 161–163 CrossRef CAS.
- E. C. da Silva Filho, J. C. P. de Melo and C. Airoldi, Carbohydr. Res., 2006, 341, 2842–2850 CrossRef CAS PubMed.
- O. Green, S. Grubjesic, S. Lee and M. A. Firestone, Polym. Rev., 2009, 49, 339–360 CrossRef CAS PubMed.
- S. Soll, Q. Zhao, J. Weber and J. Yuan, Chem. Mater., 2013, 25, 3003–3010 CrossRef CAS.
- Z.-L. Xie, X. Huang and A. Taubert, Adv. Funct. Mater., 2014, 24, 2837–2843 CrossRef CAS PubMed.
- J. Guo, L. Qiu, Z. Deng and F. Yan, Polym. Chem., 2013, 4, 1309–1312 RSC.
- L. Han, S.-W. Park and D.-W. Park, Energy Environ. Sci., 2009, 2, 1286–1292 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and some characterization of cellulosic PILs. See DOI: 10.1039/c5ra05667e |
| This journal is © The Royal Society of Chemistry 2015 |