A unique polymeric gel by thiol–alkyne click chemistry†
Mutyala Naidu Ganivada,
Pawan Kumar and
Raja Shunmugam*
Polymer Research Centre, Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, India. E-mail: sraja@iiserkol.ac.in
First published on 26th May 2015
Abstract
Poly(ethylene glycol) functionalized with tetra-acetylene (PTETACT) and pentaerythritol (3-mercaptopropionic acid) (PETM) are cross-linked by a thiol–yne reaction to create robust, tuneable networks. A new class of unique gels is produced by gelations in various organic solvents as well as water. This is the first report of creating 3D gel networks by a thiol–alkyne reaction in the presence of triethylamine under moderate temperature.
Gels can be classified in two types: either by the bonds present in the network or by the nature of the liquid component used for gelation. The gel shall be called a hydrogel if the liquid component is water1,2 or an organogel in the case of an organic liquid component.3,4 Similarly, based on the nature of the bond involved in the gelation process, a gel can be classified. A physical gel is held by physical forces of attraction for example either by van der Waals forces or hydrogen bonds.5–7 However chemical gels are held by covalent bonds.8,9 This report explores the possibility of making unique organo/chemical-gels without using a metal catalyst but by simple thiol–alkyne click chemistry.
Organogel is a solid material in which a liquid organic phase entrapped in a three-dimensionally cross-linked network. These gels are finding vast applications including electronics, medical, sports and household.10–13 Synthesis of this type of organogels can be achieved by following two strategies; either by using high molecular weight polymers as basic material or by using low molecular weight polymers in combination with a chain extender. Presently, there are few methods to make a variety of organogels, which are well documented in the literature.14 But the problem associated with these gels is the removal metal catalyst or other cross-linker that has been used to generate the gel network. Recently, one type of chemistry that is emerging as a potential candidate for making chemical gels, without the use of a catalyst, is thiol–ene chemistry.
Thiol–ene click chemistry has emerged remarkably in popularity over the past few years due to its numerous advantages. This Michael addition reaction is highly efficient and applicable to a wide variety of functional groups.15 Due to high nucleophilicity of the sulfhydryl moiety, thiol–ene coupling proceeds under physiological conditions.16,17 The advantage of the thioether linkage is very stable under physiological conditions and resists a strong basic or acidic environment. In the literature, thiol–ene reaction of multifunctional thiols with multifunctional enes is well documented in detail for the preparation of chemical gels with different types of functionality.18 For example, gels that are useful in medical applications,19–21 low gas permeable membrane, dental and optical applications to mention few.22,23 However, thiol–yne reactions to make 3D network is not explored in detail. Particularly, thiol–yne reactions without catalyst or UV light but in presence of simple amine and moderate temperature are so far not explored in the literature.
In this communication, we report poly(ethylene glycol) functionalized with tetra-acetylene (PTETACT) and pentaerythritol (3-mercaptopropionic acid) (PETM) are forming an interesting 3D network in presence of triethylamine under moderate temperature. The electron density on the ene (or yne) moiety is directly related to the thiol–ene free radical reaction rate.24 Hence, we have predicted that the thiol–yne reaction between PTETACT and PTEM would generate an interesting 3D network due to the enriched electron density associated with PTETACT.
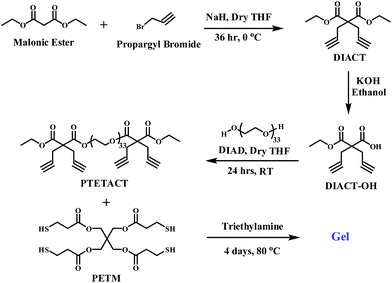
The main objective of this study is to develop an effective method to prepare polymeric gels using thiol–alkyne click chemistry, which has, so far, not been much explored. Towards this goal, PTETACT and PETM were used in the formation of polymeric gels as shown in Scheme 1. PTETACT was first synthesised in three steps (Scheme 1) using simple reaction conditions. DIACT was prepared by treating propargyl bromide with the malonic ester in presence of sodium hydride. The crude product was purified by column chromatography, which gave DIACT as a colourless crystal with 90% yield. The formation of product was confirmed by 1H NMR (Fig. S1†), 13C NMR (Fig. S2†), MASS and FT-IR spectroscopy. Selective mono ester hydrolysis of DIACT produced DIACT-OH as colourless crystal with 90% yield. In 1H NMR spectroscopy (Fig. S3†), the appearance of new signal at δ 8.35 ppm, which was responsible for carboxylic acid protons, confirmed the formation of the DIACT-OH. It was further confirmed by the 13C NMR (Fig. S4†), MASS and FT-IR spectroscopy.
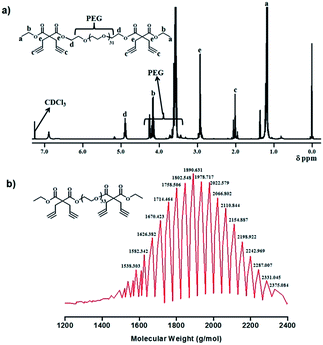
Finally to prepare PTETACT, the efficient Mitsunobu coupling reaction was employed.25 Coupling reaction between poly(ethylene glycol) (PEG-OH) (M.W. = 1450 g mol−1) (Fig. S5†) and excess of DIACT-OH in the presence of Ph3P and DIAD produced PTETACT. The formation of product was confirmed by the 1H NMR spectroscopy and MALDI-TOF as shown in Fig. 1. The measured m/z values in MALDI analysis was agreeing with the desired structure.
After the successful synthesis of PTETACT, it's click chemistry reaction with pentaerythritol (3-mercaptopropionic acid) (PETM) was explored. But in the literature, most of the thiol and acetylene click reactions were performed under the UV light. However, in our experiments, the click reaction was performed, for the first time, in presence of triethylamime (TEA) under moderate heating conditions which produced interesting gel (Scheme 1). In a typical reaction, the click reaction of PTETACT with PETM was performed in presence of triethylamime (TEA) at 80 °C temperature for 4 days. Formation of the gel was first characterized by FT-IR spectroscopy and later confirmed by rheology experiment. The thermogravimetric analysis study under N2 condition confirmed thermal stability of polymeric gel upto 320 °C (Fig. S6†).
Fig. 2 shows the FT-IR spectra of the PTETACT, PETM and Gel (PTETACT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PETM) (1
PETM) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) before and after cross-linking. It was clearly evident from the FT-IR of the freeze–dried gels that the S–H peak of free thiol at 2568 cm−1 observed in PETM was completely absent in Gel. This was clearly responsible for a successful click event where a C–S–C linkage was formed between PETM and PTETACT (Fig. 3). From the FT-IR spectroscopy, it was confirmed that the thiol–alkyne reaction did produce the expected gel.
3) before and after cross-linking. It was clearly evident from the FT-IR of the freeze–dried gels that the S–H peak of free thiol at 2568 cm−1 observed in PETM was completely absent in Gel. This was clearly responsible for a successful click event where a C–S–C linkage was formed between PETM and PTETACT (Fig. 3). From the FT-IR spectroscopy, it was confirmed that the thiol–alkyne reaction did produce the expected gel.
The gel formation was first observed by the vial inversion experiment (Fig. 4a). To find out the best feed ratio of PETM and PTETACT to get the strong gel, different combinations of the mol ratios of PTETACT and PETM were tried. It was very interesting to note that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratios of PTETACT and PETM gave the strongest gel. If the ratio was not 1
3 molar ratios of PTETACT and PETM gave the strongest gel. If the ratio was not 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, then different type of gels like, weak, very weak or liquid gels were observed (Table S1†). This prompted us to propose the mechanism as shown in the Fig. 3a. To measure the mechanical strength of the gels produced with various feed ratios, rheology experiments were performed using a steel parallel plate with 40 mm diameter at 25 °C with 1.0 mm gap spacing for all gel samples. Measurements of storage modulus (G′) and loss modulus (G′′) versus %strain of gel materials were first performed to understand their mechanical strength.
3, then different type of gels like, weak, very weak or liquid gels were observed (Table S1†). This prompted us to propose the mechanism as shown in the Fig. 3a. To measure the mechanical strength of the gels produced with various feed ratios, rheology experiments were performed using a steel parallel plate with 40 mm diameter at 25 °C with 1.0 mm gap spacing for all gel samples. Measurements of storage modulus (G′) and loss modulus (G′′) versus %strain of gel materials were first performed to understand their mechanical strength.
The greater value of G′ than G′′ over the measured frequency range for the gel obtained from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratios of PTETACT and PETM, demonstrated the maximum mechanical behaviour for a typical cross-linked 3D network (Fig. 4d). As expected, the differences in G′ and G′′ for the weaker gels were not much as shown in Fig. S7 and S8.†
3 molar ratios of PTETACT and PETM, demonstrated the maximum mechanical behaviour for a typical cross-linked 3D network (Fig. 4d). As expected, the differences in G′ and G′′ for the weaker gels were not much as shown in Fig. S7 and S8.†
After standardizing the conditions for the strong gel formation, swelling capacity of the gel was explored.26 Swelling ratio was monitored for 24 hour with interval of time (Table S2 and S3†). From the given experimental conditions, it was observed that the swelling did occur in organic solvents such as DMSO, DMF, DCM, chloroform and water within an hour (Fig. 4b). The maximum swelling occurred in DCM, followed by chloroform. We believed that by increasing the PEG content, the swelling in water would improve a lot. It was very interesting to note that the gel never showed swelling behaviour in toluene and hexane.
Finally, microstructures of both organogels as well as hydrogels were studied with scanning electron microscopy (SEM). In general, all gels showed a continuous gel structure under the SEM. It was clearly evident from the SEM studies that a large number of porous structures could be seen in the case of organogels (Fig. 4c). This was quite obvious since the microporous gels were obtained using polymers cross-linked at the chain ends. The maximum as well as uniform porous were observed in the case of gels swollen in DCM and CHCl3 solvents (Fig. S9b and c†). The porous structure also observed in the case of gels swollen in CHCl3 and DMF (Fig. S9c and d†). Interestingly the morphology totally changed in the case of gels swollen in DMSO solvent. Instead of porous structure, a fibre like morphology (Fig. S9e†) was observed in the case of gels swollen in DMSO. Surprisingly, there was a uniform pattern observed in the case of hydrogels (Fig. S9f†). Since the swelling was not that effective in the case of H2O, the pores observed were not too large. More importantly, it was very interesting to note that the gels kept in toluene and hexane never swollen so their morphology of the surface as well as interior never changed at all.
In conclusion, thiol–yne reaction of PTETACT and PETM is proved to be an efficient way for creating chemical gels. It is important to note that the reaction is not inhibited by metal catalyst or other curators since the method is not depending on the use of any heavy metal catalyst. FT-IR spectroscopy analysis of the cross-linked thiol–yne gel network demonstrates that the reaction between thiol and yne in this particular system is extremely efficient. The triple bond conversion of the thiol–yne networks are all identical to the maximum possible conversion calculated from the thiol to yne feed-ratio. It is interesting to note that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratios of PTETACT and PETM produce the strongest gel which is confirmed by rheology data. The highest swelling is observed in DCM. The SEM image of the swollen gel in DCM shows uniform pores present in the gel. We believe that increase in PEG content would increase the hydrogel capabilities. The non-swelling property of the gel in hexane and toluene has prompted us to envisage the gel's application in removing chlorinated solvents from hydrocarbon solvents.
3 molar ratios of PTETACT and PETM produce the strongest gel which is confirmed by rheology data. The highest swelling is observed in DCM. The SEM image of the swollen gel in DCM shows uniform pores present in the gel. We believe that increase in PEG content would increase the hydrogel capabilities. The non-swelling property of the gel in hexane and toluene has prompted us to envisage the gel's application in removing chlorinated solvents from hydrocarbon solvents.
Acknowledgements
GMN thanks UGC for the research fellowship and RS thanks DST, New Delhi for Ramanujan Fellowship and IISER-Kolkata for the infrastructure. Mr Kashinath from Carl Zeiss India, is acknowledged for the help in capturing quality SEM pictures.Notes and references
- R. E. Webber and C. Creton, Macromolecules, 2007, 40, 2919–2927 CrossRef CAS.
- M. P. Lutolf, Nat. Mater., 2009, 8, 451–453 CrossRef CAS PubMed.
- E. A. Wilder, C. K. Hall, S. A. Khan and R. J. Spontak, Langmuir, 2003, 19, 6004–6013 CrossRef CAS.
- M. Watase, Y. Nakatani and H. Itagaki, J. Phys. Chem. B, 1999, 103, 2366–2373 CrossRef CAS.
- E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195–6214 RSC.
- M. Djabourov and P. Papon, Polymer, 1983, 24, 537–542 CrossRef CAS.
- R. Shunmugam, C. E. Smith and G. N. Tew, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2601–2608 CrossRef CAS PubMed.
- C. D. Pritchard, T. M. O'Shea, D. J. Siegwart, E. Calo, D. G. Anderson, F. M. Reynolds, J. A. Thomas, J. R. Slotkin, E. J. Woodard and R. Langer, Biomaterials, 2011, 32, 587–597 CrossRef CAS PubMed.
- Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485–487 CrossRef CAS.
- S. S. Babu, K. K. Kartha and A. Ajayaghosh, J. Phys. Chem. Lett., 2010, 1, 3413–3424 CrossRef CAS.
- M. N. Ganivada, V. N. Rao, H. Dinda, P. Kumar, J. D. Sarma and R. Shunmugam, Macromolecules, 2014, 47, 2703–2711 CrossRef CAS.
- K. R. Kamath and K. Park, Adv. Drug Delivery Rev., 1993, 11, 59–84 CrossRef CAS.
- J. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- K. Zhang, M. A. Lackey, J. Cui and G. N. Tew, J. Am. Chem. Soc., 2011, 133, 4140–4148 CrossRef CAS PubMed.
- A. Gress, A. Volkel and H. Schlaad, Macromolecules, 2007, 40, 7928–7933 CrossRef CAS.
- A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995–8997 CrossRef CAS PubMed.
- C. E. Hoyle, T. Y. Lee and T. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301–5338 CrossRef CAS PubMed.
- R. T. C. Cleophas, M. Riool, H. C. Q. V. Ufford, S. A. J. Zaat, J. A. W. Kruijtzer and R. M. J. Liskamp, ACS Macro Lett., 2014, 3, 477–480 CrossRef CAS.
- A. Song, A. A. Rane and K. L. Christman, Acta Biomater., 2012, 8, 41–50 CrossRef CAS PubMed.
- N. M. Matsumoto, D. C. Gonezalez-Toro, R. T. Chacko, H. D. Maynard and S. Thayumanavan, Polym. Chem., 2013, 4, 2464–2469 RSC.
- A. A. Aimetti, A. J. Machen and K. S. Anseth, Biomaterials, 2009, 30, 6048–6054 CrossRef CAS PubMed.
- T. Ono, T. Sugimoto, S. Shinkai and K. Sada, Nat. Mater., 2007, 6, 429–433 CrossRef CAS PubMed.
- Y. Zhou, N. Sharma, P. Deshmukh, R. K. Lakhman, M. Jain and R. M. Kasi, J. Am. Chem. Soc., 2012, 134, 1630–1641 CrossRef CAS PubMed.
- H. Kim, J. V. Olsson, J. L. Hedrick and R. M. Waymouth, ACS Macro Lett., 2012, 1, 845–847 CrossRef CAS.
- K. Zhang, J. Cui, M. Lackey and G. N. Tew, Macromolecules, 2010, 43, 10246–10252 CrossRef CAS.
- S. G. Roy, U. Haldar and P. De, ACS Appl. Mater. Interfaces, 2014, 6, 4233–4424 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure, TGA and additional analytical data. This material is available free of charge via the Internet at DOI: 10.1039/c5ra06339f |
| This journal is © The Royal Society of Chemistry 2015 |