Palladium-catalyzed desulfurative Sonogashira cross-coupling reaction of 3-cyano assisted thioamide-type quinolone derivatives with alkynes†
Abstract
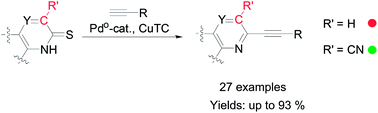
A Pd-catalyzed Cu-mediated desulfurative Sonogashira cross-coupling reaction of thioamide-type quinolone derivatives was proposed for the construction of Csp2–Csp bonds. Alkynylated quinoline derivatives can be easily synthesized in moderate to excellent yields. The mechanism and effect of the 3-cyano on the reaction were also discussed.

 Please wait while we load your content...
Please wait while we load your content...