Fabrication and modeling of an ultrasensitive label free impedimetric immunosensor for Aflatoxin B1 based on poly(o-phenylenediamine) modified gold 3D nano electrode ensembles
Abstract
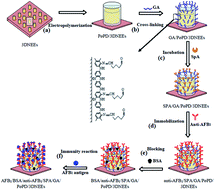
An ultrasensitive label free impedimetric immunosensor for AFB1 detection was fabricated based on poly(o-phenylenediamine) (PoPD) electropolymerized film modified gold three dimensional nanoelectrode ensembles (3DNEEs). The nanoelectrode ensembles were fabricated by template synthesis in track etched polycarbonate (PC) membranes. Scanning electron microscopy and X-ray diffraction were used for the evaluation of the properties of 3DNEEs. Anti-AFB1 was directionally immobilized on Staphylococcus protein A (SpA) with orientation functions. Cyclic voltammetry and electrochemical impedance spectroscopy were employed to characterize the fabrication process and optimize working conditions. The interface model of molecular recognition was constructed and reasonably interpreted. The detection limit was 0.019 ng mL−1 (S/N = 3). The linear detection concentration range of AFB1 was from 0.04 to 8.0 ng mL−1.

 Please wait while we load your content...
Please wait while we load your content...