A simple and dual responsive efficient new Schiff base chemoreceptor for selective sensing of F− and Hg2+: application to bioimaging in living cells and mimicking of molecular logic gates†
Abstract
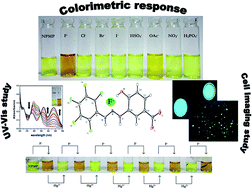
A novel colorimetric hydrazine-functionalized Schiff base chemoreceptor, NPMP, was synthesized following a simple one-step Schiff base condensation pathway. NPMP showed selective colorimetric change from faint yellow to yellowish orange in the presence of biologically ubiquitous fluoride (F−). It also showed a ‘turn off’ fluorescent response in the presence of F− that could effectively distinguish it from all anions tested except acetate. Acetate (OAc−) caused a weak response, while other anions like chloride, bromide, iodide, phosphate, hydrogen sulfate and nitrate did not have any observable effect on the NPMP receptor (E)-4-nitro-2-((2-(perfluorophenyl)hydrazono)methyl)phenol. Recognition of F− in the presence of NPMP can be explained in light of multiple H-bonding interactions, as well as acid–base interactions between host receptor and guest F−. Interestingly, NPMP also showed enormous potential as a staining agent in determining the presence of low levels of intracellular fluoride. Moreover, it was found that in NPMP⋯F− solutions, incorporation of Hg2+ showed observable optical changes, revealing that this compound is a smart material. Optical responses of NPMP can mimic a molecular logic gate (INHIBIT gate). This can be interpreted as a combination of an AND gate with a NOT function. It also represented a potential ‘Write–Read–Erase–Read’ memory function reflecting multi-writing ability.

 Please wait while we load your content...
Please wait while we load your content...