Thermosensitive mixed shell polymeric micelles decorated with gold nanoparticles at the outmost surface: tunable surface plasmon resonance and enhanced catalytic properties with excellent colloidal stability†
Tao Yin,
Xue Liu,
Jianzu Wang,
Yingli An,
Zhenkun Zhang* and
Linqi Shi*
Key Laboratory of Functional Polymer Materials of Ministry of Education, Institute of Polymer Chemistry, College of Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin, 300071, China. E-mail: zkzhang@nankai.edu.cn; shilinqi@nankai.edu.cn
First published on 8th May 2015
Abstract
Hybrid particulate composites consisting of noble metal nanoparticles (NPs) and polymeric particles have attracted intensive interest, due to the possibility of combining the precious optical and catalytic properties of the former with the stimuli responsiveness and biocompatibility of the latter. However, it is challenging to prepare hybrid particles that simultaneously have tunable optical and catalytic properties as well as excellent colloidal stability. In the current work, we report a strategy for such hybrid particles, through covalently decorating the outmost surface of mixed shell polymeric micelles (MSPMs) with gold NPs. For this, two block polymers, poly(ε-caprolactone)-block-(ethylene glycol) (PCL-b-PEG) and poly(ε-caprolactone)-block-poly(N-isopropylacrylamide) (PCL-b-PNIPAM), were prepared by ring-opening polymerization and reversible addition fragmentation chain transfer (RAFT) polymerization, respectively. Co-self-assembly of the two block polymers result in MSPMs with a PCL core and a mixed shell consisting of PEG and PNIPAM. At the end of each PNIPAM chain in the shell, thiol groups are introduced to act as anchors for the in situ formation of gold NPs. The number density of the gold NPs is conveniently tuned through varying the relative amount of PEG/PNIPAM in the micellar shell. Reversible shrinking and extension of the PNIPAM chains regulated by temperature can be used to tune the interparticle distance of the gold NPs, while the whole hybrid particles are stabilized by the stretched PEG chains. The hybrid polymeric micelles exhibit thermoresponsive surface plasmon resonance and enhanced catalytic properties as well as excellent colloidal stability.
Introduction
Noble metal nanoparticles (NPs) made of gold, silver, platinum, etc., have been extensively exploited due to their precious optical and catalytic properties and found applications in a wide spectrum of fields such as catalysis, photoelectric devices and biomedicine.1–6 At the same time, their organic counterparts, mostly polymeric nanoparticles often with a much larger size, have been playing critical roles in manmade nanoscale platforms for drug delivery, bio-imaging and mimics of biological functions.7–11 Nowadays, the preparation of both kinds of nanoparticles, often being pursued separately, has made significant progress. Especially, in the cases of polymeric NPs based on polymeric micelles, the self-assembly of block polymers offers versatile possibilities to fabricate nano-assemblies with rich morphologies and internal architectures.12–14 Naturally, combining the two kinds of particles together to create hybrid nanoparticles has come into the sight of many researchers.15–21 On one hand, polymeric NPs have been wisely recruited as templates either for the formation of metal NPs,22–26 or for the three dimensional arrangement of preformed metal NPs.17,27–30 On the other hand, installing polymeric NPs with metal NPs can impart novel functional properties upon the otherwise inert polymeric materials to realize functional integration. A large library of hybrid NPs has been prepared and the NPs have found many novel applications in fields such as heterogeneous catalysis, efficient cancer diagnosis and treatment, etc.31–37In hybrid NPs consisting of metal and polymeric NPs, the metal NPs can either be loaded into the core,36,38,39 homogenously distributed inside whole particles,23,26 or located on the peripheral surface of the polymeric particles.16–18,29 Hybrid particles in the last case have a core–shell architecture and are especially attractive for several reasons. First of all, the surface exposed metal NPs can be accessed easily by species in the surrounding media which is important for catalysis and sensing.25,29,40 Furthermore, it is possible to control the inter-particle distances of metal NPs loaded at the outmost surface, which are well known to be related to the electronic and magnetic properties of the nanoparticles.18,41,42 With such goals in mind, stimuli responsive polymeric components such as thermo-responsive poly(N-isopropylacrylamide) (PNIPAM), and pH sensitive poly(vinylpyridine) (PVP), have been used as the anchors for the metal NPs.41–43 Environmental stimuli, such as the temperature or pH can be used to induce reversible collapse/swelling of the polymeric components. Accordingly, the spatial position of the loaded metal NPs can also be fine-tuned, resulting in tunable surface plasmon resonance (SPR), surface enhanced Raman scattering (SERS) or catalytic behavior.41–43 In these examples, an often neglected issue is the colloidal stability of the hybrid particles, i.e., the hybrid nanoparticles can remain discretely separated while performing their function under environmental stimuli. Aggregation under working conditions will compromise the performance of the loaded metal NPs. Such a situation might occur for hybrid particles with gold NPs installed onto a temperature or pH responsive polymeric shell.44,45 For instance, a collapsed polymeric component such as PNIPAM is hydrophobic which would introduce attraction between the hybrid particles, often resulting in irreversible aggregation.44 Alternatively, the interparticle distance can be controlled by the density of the metal NPs loaded onto the peripheral surface of the polymeric particles.29 If the size of the polymeric template is set, increasing the density of metal NPs means decreasing the interparticle distance. However, a convenient way to control the density of the metal NPs with certain precision is desperately needed. To the best of our knowledge, only few works have realized such goals.
In the current work, we shall decorate mixed shell polymeric micelles (MSPMs) with gold NPs through the in situ reduction of a gold precursor, to prepare hybrid polymeric micelles with a thermoresponsive SPR and catalytic properties as well as an enhanced colloidal stability. MSPMs refer to core–shell micelles with at least two types of polymeric chains in the micellar shell. One of the chains in the micellar shell can be stimuli responsive, which offers an extremely flexible way to fine-tune the surface properties of the polymeric micelles. For the current work, we shall design MSPMs with a poly(ε-caprolactone) (PCL) core and a mixed shell consisting of poly(ethylene glycol) (PEG) and poly(N-isopropylacrylamide) (PNIPAM) through the co-assembly of poly(ε-caprolactone)-b-PEG (PCL-b-PEG) and PCL-b-PNIPAM (Scheme 1). The end of each PNIPAM chain at the outmost of the micelles is coupled with a thiol group which is naturally derived from the reduction of the thiocarbonylthio group of the macro chain transfer agent for the reversible addition fragmentation chain transfer (RAFT) polymerization. The in situ reduction of a gold precursor can install the outmost surface of such MSPMs with gold NPs which are stabilized by the thiol groups at the end of the PNIPAM chains. Two advantages are unique to the current strategy. First of all, the co-assembly of two kinds of block copolymers offers the flexibility to control the density of the surface thiol groups which are the main anchoring point of the gold particles. In this way, the number density of the loading gold NPs can also be varied. Furthermore, the interparticle distance of the gold NPs can be fine-tuned by heating and cooling to induce the shrinking/extension of the PNIPAM chains, resulting in tunable SPR catalytic properties. Another advantage is that the whole hybrid particles will be stabilized by the stretched PEG chains in the micellar shell, endowing the current system with excellent colloidal stability under working conditions.
 | ||
| Scheme 1 Schematic illustration of the procedure for the preparation of the hybrid mixed shell polymeric micelles decorated with gold NPs on their outmost surface. | ||
Experimental
Materials
HAuCl4·3H2O (>99.9%) and NaBH4 (>98.9%) were obtained from J&K (J&K Scientific Ltd., Beijing, China) and used without further purification. The two block polymers, poly(ε-caprolactone)88-block-(ethylene glycol)113 (PCL88-b-PEG113) and poly(ε-caprolactone)83-block-poly(N-isopropylacrylamide)90 (PCL78-b-PNIPAM90), were prepared as reported in our previous work.46 The subscripts in the block copolymers designate the degrees of polymerization. All the organic solvents were dried following the standard procedure.Preparation of mixed shell polymeric micelles (MSPMs)
First, 2 mg of the mixture of the two block copolymers, PCL-b-PEG and PCL-b-PNIPAM with different mass ratios was dissolved in 1.5 mL of anhydrous THF. The resulting polymer solution was then added dropwise into 13 mL of ultrapure water under vigorous stirring. The solution became opalescent eventually, indicating the formation of micelles. The solution was further stirred overnight and dialyzed against water to remove THF. The final concentration of the micelles was 0.1 mg mL−1. In a similar way, micelles consisting of only PCL-b-PNIPAM were also prepared.Preparation of polymeric micelles decorated with gold NPs
To prepare hybrid polymeric micelles decorated with gold NPs, the first step is to transform the thiocarbonylthio group at the end of the PNIPAM chains of the micelles into thiol groups. For this, to a 2 mL suspension of the polymeric micelles, 0.5 mL of NaBH4 solution (1 M) was added. The mixture was stirred for 48 h in the dark and under a nitrogen atmosphere. The final mixture was then dialyzed in a dialysis tube (3.5 kDa MWCO, Spectrum) against deoxygenated water under a nitrogen atmosphere to avoid oxidation of the thiol groups into disulfide bonds. The dialyzed mixture was concentrated to 2 mL by ultrafiltration centrifugation in an ultrafiltration tube (4 kDa MWCO, Millipore) at a centrifuge speed of 4000 rpm. To the suspension maintained at 37 °C and purged with nitrogen, 400 μL of aqueous solution of HAuCl4·4H2O (1.6 mg mL−1) was added under stirring. The mixture was further stirred for another 5 h and then 200 μL of NaBH4 solution (4 mM) was added. The mixture was stirred for 24 h. The final particles were washed with water three times by centrifuging and redispersion.Catalytic reduction of p-nitrophenol by hybrid polymeric micelles
The catalytic reduction was carried out in a standard quartz cuvette of 1 cm path length. Aqueous solutions of p-nitrophenol (350 μL, 0.1 mM) and NaBH4 (150 μL, 10 mM) were mixed together, the pH of which was kept at 10 using 1 M NaOH solution, if necessary. After the polymeric micelles decorated with gold NPs were introduced to the mixture, the time-dependent absorption spectra were recorded on a TU-8119 UV-vis spectrophotometer. To study the temperature effect, the temperature of the sample holder was controlled with an accuracy of 0.01 °C by an HAAKE A28 external circulating water bath installed with an HAAKE SC 100 controller (Thermo Scientific). In a similar way, pure gold NPs stabilized by citrate were also assessed.Characterization
Transmission electron microscopy (TEM) measurements were performed on a Philips T20ST electron microscope at an acceleration voltage of 100 kV. The TEM samples were negatively stained by uranyl acetate 2% ethanol solution for the bare polymeric micelles while the gold NP decorated ones were directly checked without any pretreatment. Dynamic light scattering (DLS) measurements were conducted on a laser light scattering spectrometer (BI-200SM, Brookhaven) equipped with a digital correlator (BI-9000AT, Brookhaven) at 636 nm at given temperatures. All the samples were obtained by filtering through a 0.45 μm Millipore filter. X-ray photoelectron spectroscopy (XPS) measurements were performed with a Kratos Axis Ultra DLD multi-technique X-ray photoelectron spectrometer (Kratos Analytical Ltd., UK).Results and discussion
Preparation of mixed shell polymeric micelles
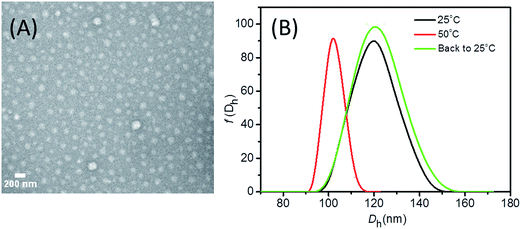
The two block polymers, PCL83-b-PNIPAM90 and PCL78-b-PEG113, were prepared as reported in our previous publication.46 A tailor-designed PCL coupled with a thiocarbonylthio group at one of the chain ends was used as the macro chain transfer agent for reversible addition fragmentation chain transfer (RAFT) polymerization of NIPAM, resulting in PCL-b-PNIPAM with the thiocarbonylthio group sitting at the end of the PNIPAM block (insets of Scheme 1). Following our previous method, co-assembly of PCL-b-PNIPAM and PCL-b-PEG resulted in mixed shell polymeric micelles (MSPMs) with a PCL core and a mixed PEG/PNIPAM shell.47 The relative ratio of the PEG/PNIPAM in the micellar shell can be adjusted by controlling the relative amount of the two block polymers in the starting materials. The MSPMs were characterized by dynamic light scattering (DLS) and negative staining TEM (Fig. 1). The average hydrodynamic diameter (〈Dh〉) of the micelles has a typical value of 120 nm and a narrow size distribution at room temperature (Fig. 1B). When heating up to more than 32 °C, i.e. the lower critical solution temperature (LCST) of the PNIPAM chains, 〈Dh〉 decreases to 105 nm while it still has a narrow distribution (Fig. 1B). As already confirmed in previous work by us and others, at T > LCST, the PNIPAM chains in the micellar shell can collapse from the extended and hydrated state onto the micellar core and form hydrophobic domains while the whole micelles are still colloidally stable due to the steric hindrance offered by the hydrated PEG blocks (step 4 in Scheme 1).47,48 By cooling, the collapsed PNIPAM chains turn into the extended hydrophilic state and the size of the micelles recovered (Fig. 1B). In this way, if there are functional species at the end of the PNIPAM chains in the shell, their spatial position in three dimensions is expected to be adjusted conveniently by temperature.49In situ formation of gold nanoparticles on the surface of MSPMs
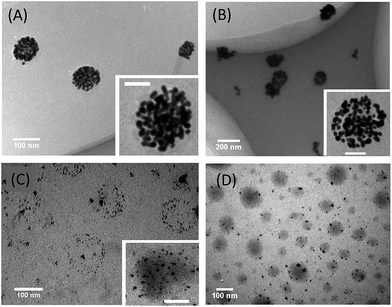
To decorate the outmost surface of the MSPMs with gold NPs, the thiocarbonylthio end groups at the end of PNIPAM of the MSPMs were first cleaved into thiol groups by the classic NaBH4 reduction (Scheme 1).45,50 Into the same mixture in the presence of NaBH4, H4AuCl4, the precursor for the gold NPs, was added which was then turned into gold NPs. By optimizing the amount of the precursor relative to the micelle templates, well-dispersed discrete hybrid micelles with gold NPs decorating their outmost surface were obtained which will be referred to as MSPM@AuNPs (Fig. 2). XPS survey spectra of MSMPs with or without gold NPs indicate that a new peak corresponding to Au (4f) appears in the case of MSMPs with gold NPs (Fig. S1†). In the whole procedure, no external ligand is needed. NaBH4 is a strong reducing agent that leads to a fast rate of nucleation and smaller gold nanoparticles. As soon as the gold nanoparticles are produced, the surface-exposed thiol groups at the end of PNIPAM in our micelles are chemisorbed onto the surfaces of the in situ formed gold nanoparticles, which will stabilize the gold nanoparticles, on one hand. On the other hand, the surface-exposed thiol groups at the end of PNIPAM act as anchor points to limit the location of gold NPs at the micellar surface.45,50,51 Several PNIPAM chains bind to one NP (Scheme 1).51 The whole hybrid particle has an average size of 130 nm, which is slightly larger than the bare MSPM templates (Fig. 3B). The as-prepared hybrid particles can maintain colloidal stability under refrigerated conditions for several months without precipitation. Control experiments were performed with similar MSPMs without surface thiol groups and no gold NP decoration occurred.One of the advantages of the current strategy is that the surface thiol groups of the micellar template can be controlled by changing the relative amount of the two block copolymers during self-assembly. By using polymeric micelles with a similar size while varying the amount of surface thiol groups, the number of gold NPs on the outmost surface of the MSPM can be fine-tuned (Fig. 2). In the case of the simple micelles formed by PCL-b-PNIPAM alone, the surface of the micelle has the highest amount of thiol groups. Accordingly, the micelle is decorated with gold NPs which are densely packed (Fig. 2A). In the case of MSPMs, the amount of the thiol in the shell can be reduced by increasing the ratio of PCL-b-PEG. The number of gold NPs on the micellar surface also decreases. For instance, MSPMs with a PEG/PNIPAM mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 are still decorated with many gold NPs but increased inter-particle distances and spare spaces are clearly discernable (inset of Fig. 2B). After further increasing the PEG/PNIPAM ratio to 1
9 are still decorated with many gold NPs but increased inter-particle distances and spare spaces are clearly discernable (inset of Fig. 2B). After further increasing the PEG/PNIPAM ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the micelles are only sparsely decorated with gold NPs (Fig. 2C). In the case of micelles with a PEG/PNIPAM ratio of 1
4, the micelles are only sparsely decorated with gold NPs (Fig. 2C). In the case of micelles with a PEG/PNIPAM ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, there are only several gold NPs on the surface of the micelles (Fig. 2D). Therefore, the current strategy offers a very convenient way to control the density of the gold NPs on the surface of the hybrid polymeric particles.
1, there are only several gold NPs on the surface of the micelles (Fig. 2D). Therefore, the current strategy offers a very convenient way to control the density of the gold NPs on the surface of the hybrid polymeric particles.
The morphology of the gold NPs exhibits some unique characteristics which is dependent on the density of the gold particles and is also different from the usual spherical shape of gold NPs obtained in the presence of organic ligands such as citrate (Fig. S3A in ESI†). As shown in the insets of Fig. 2A and B, many of the gold NPs have a dumbbell like shape which probably originates from the fusion of two spherical gold NPs.52 Discrete spherical gold NPs, with an average diameter of 8 nm, become dominant with decreasing thiol groups in the micellar shell (Fig. 3C and D).
Thermo-responsive on-particle aggregation behavior of gold NPs without losing colloidal stability of the hybrid micelles
As discussed before, the terminal functional groups of the PNIPAM chains in the mixed shell of the micelles can change their position with the shrinking and extension of the PNIPAM chains under thermal stimuli. It is therefore expected that the gold NPs at the terminal of the PNIPAM chains can also change their three dimensional position and inter-particle distance reversibly in a similar way. In the case of micelles with a PEG/PNIPAM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the gold NPs reside at the outmost surface with spare spaces which are clearly revealed by TEM (Fig. 2B). Upon heating up to the LCST, the hybrid particles transformed into solid-like particles (Fig. 3A), in which the gold NPs densely pack. Although the morphology of the hybrid particles is generally in the spherical shape, their surface becomes rugged (inset of Fig. 3A). The hydrodynamic size distribution obtained from the DLS spectra gives a sharp peak with a value of 110 nm at T > LCST, lower than 130 nm for the same particle at room temperature (Fig. 4B). In addition, the color of the hybrid micellar suspension also changed from red to deep purple during heating, a clear sign indicating that the surface gold NPs have changed their interparticle distance. These results are both consistent with the fact that the gold NPs at the terminal of the PNIPAM chains become close to each other with the collapse of the PNIPAM chain during heating. In this way, the interparticle distance of the gold NPs decreases dramatically, which is beneficial for the optical properties of the particles as will be demonstrated later.
9, the gold NPs reside at the outmost surface with spare spaces which are clearly revealed by TEM (Fig. 2B). Upon heating up to the LCST, the hybrid particles transformed into solid-like particles (Fig. 3A), in which the gold NPs densely pack. Although the morphology of the hybrid particles is generally in the spherical shape, their surface becomes rugged (inset of Fig. 3A). The hydrodynamic size distribution obtained from the DLS spectra gives a sharp peak with a value of 110 nm at T > LCST, lower than 130 nm for the same particle at room temperature (Fig. 4B). In addition, the color of the hybrid micellar suspension also changed from red to deep purple during heating, a clear sign indicating that the surface gold NPs have changed their interparticle distance. These results are both consistent with the fact that the gold NPs at the terminal of the PNIPAM chains become close to each other with the collapse of the PNIPAM chain during heating. In this way, the interparticle distance of the gold NPs decreases dramatically, which is beneficial for the optical properties of the particles as will be demonstrated later.
 | ||
| Fig. 4 Surface plasmon resonance (SPR) of gold NPs templated by the mixed shell polymeric micelles. (A) UV-vis absorption spectra of the four kinds of hybrid particles in Fig. 2 as well as gold NPs stabilized by citrate. (B) UV-vis absorption spectra of the MSPM@gold NPs during heating. (C) The absorption peak versus temperature of the MSPM@gold NPs during heating/cooling. (D) Red shift behavior of the SPR of the MSPM@gold NPs during heating/cooling cycles. The MSPM@gold NPs in (B), (C) and (D) are the same as those in Fig. 2B. | ||
The narrow hydrodynamic size distribution at 40 °C in Fig. 3B also indicates that during heating, no aggregation among the hybrid particles occurs. This fact points to the excellent colloidal stability of the hybrid particles based on the MSMPs due to the steric hindrance of the stretched PEG chains. This can be also confirmed from visual inspection of the hybrid particle suspension (Fig. 3C and D). Pronounced Tyndall effects exist for the hybrid micelle particles either at room temperature or when T > LCST (MSPM@AuNPs in Fig. 3C and D). However, large aggregates form during heating the suspension of the hybrid micelles consisting of only PNIPAM and gold NPs (Fig. S2A in ESI†), which diminishes the Tyndall effect (PNIPAM@AuNPs in Fig. 3C and D).
Shown in Fig. 3B is also the hydrodynamic size distribution of MSPM@AuNPs after cooling from the heated state back to room temperature, which reveals that the cooled hybrid particles have an average hydrodynamic size matching the value of the same particles before heating. A TEM investigation of the hybrid particles cooling back to room temperature also reveals that most of the particles can reverse to the state with spare spaces between gold NPs (Fig. S2B in ESI†). These phenomena suggest that it is possible to fine tune the three dimensional arrangement and inter-particle distance of the outmost gold NPs by controlling the extension and collapse of the PNIPAM chains. A small population of particles, however, stays in the collapsed state as indicated by the shoulder peak with a maximum value similar to that of the collapsed particles (Fig. 3B). This is probably due to the interparticle fusion upon heating, during which neighboring gold NPs permanently stick together.52,53 These collapsed hybrid particles also exist in the TEM images (Fig. S2B†).
Tunable surface plasmon resonance properties of gold NPs decorated mixed micelles
One of the prestigious properties of noble metal NPs, including gold NPs, is their surface plasmon resonance (SPR) which has been exploited in many applications. Experimental and theoretical studies have confirmed that such a property critically depends on the shape, composition, morphology, and dielectric environment of particles.35,36 In recent years, the focus has been directed to arranging metal NPs into ordered suprastructures with or without templates, which can be used to tune the collective SPR properties of the metal NPs.29,36,54 In these latter cases, it is also demonstrated that the interparticle distance and localized refractive index play critical roles for responsive SPR properties. With the current strategy, we have two ways to tune the inter-particle distance of the templated gold NPs and therefore their SPR properties. First of all, the number density of the gold NPs on the outmost surface of the polymeric micelles can be controlled by varying the thiol groups on the surface of the polymeric micelles (Fig. 2). The UV-vis absorption spectra of the four kinds of hybrid particles in Fig. 2 are presented in Fig. 4A, together with citrate stabilized pure gold NPs. By increasing the number density of the gold NPs, i.e. decreasing the inter-particle distance, red shifting is observed (Fig. 4A). The absorption peak shifts from 520 nm for the pure gold NPs to 550 nm for the ones templated on the simple micelles consisting of PNIPAM alone which has the highest density of gold NPs (Fig. 2A). Therefore, co-self-assembly of PCL-b-PEG and PCL-b-PNIPAM into MSPMs is a versatile way to control the number density of the templated gold NPs, resulting in hybrid particles with tunable SPR properties.Besides the above method, we have demonstrated the reversible packing of gold NPs at the outmost surface of the micelles due to thermo-responsive collapse/extension of the PNIPAM chains. Such tunable spatial reconfiguration is naturally expected to influence the SPR properties of the templated Au NPs. Summarized in Fig. 4B are the UV-vis absorption spectra of the hybrid MSPM@AuNPs with a PEG/PNIPAM mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 upon heating from room temperature to 40 °C. A red shift occurs to the absorption peak during heating, and at ca. 32 °C, the shift levels off (Fig. 4C). In this procedure, there is a ca. 20 nm red shift, from 536 to 556 nm. Furthermore, the absorption spectra also broaden during heating. The turning point at 32 °C coincides with the LCST of PNIPAM chains in the shell at which the PNIPAM chains will collapse. Pure gold NPs stabilized by citrate exhibit no such temperature dependent behavior (Fig. S3 in ESI†). Therefore, the red-shift and broadening of the UV-vis spectra at T > 32 °C should be due to particle–particle coupling effects induced by the on-particle aggregation of the templated gold NPs when the collapse of the PNIPAM chains reduces inter-particle distances of the gold NPs. Upon cooling, the absorption peaks recover (Fig. 4C). It is noticed that a pronounced hysteresis occurs during cooling (Fig. 4C). The heating/cooling cycles can be performed for several cycles (Fig. 4D). At later stages of the cycles, the absorption peaks after cooling gradually increase and the degree of red-shift decreases (Fig. 4D). As also indicated by DLS in Fig. 3B, some hybrid particles stay in the collapsed state after cooling back to room temperature. It is reckoned that neighboring gold NPs might fuse together when being brought close to each other during heating since the gold NPs are only stabilized by the end –SH groups of the PNIPAM chains. Similar inter-particle fusion phenomena have also been suggested by others.52,53
9 upon heating from room temperature to 40 °C. A red shift occurs to the absorption peak during heating, and at ca. 32 °C, the shift levels off (Fig. 4C). In this procedure, there is a ca. 20 nm red shift, from 536 to 556 nm. Furthermore, the absorption spectra also broaden during heating. The turning point at 32 °C coincides with the LCST of PNIPAM chains in the shell at which the PNIPAM chains will collapse. Pure gold NPs stabilized by citrate exhibit no such temperature dependent behavior (Fig. S3 in ESI†). Therefore, the red-shift and broadening of the UV-vis spectra at T > 32 °C should be due to particle–particle coupling effects induced by the on-particle aggregation of the templated gold NPs when the collapse of the PNIPAM chains reduces inter-particle distances of the gold NPs. Upon cooling, the absorption peaks recover (Fig. 4C). It is noticed that a pronounced hysteresis occurs during cooling (Fig. 4C). The heating/cooling cycles can be performed for several cycles (Fig. 4D). At later stages of the cycles, the absorption peaks after cooling gradually increase and the degree of red-shift decreases (Fig. 4D). As also indicated by DLS in Fig. 3B, some hybrid particles stay in the collapsed state after cooling back to room temperature. It is reckoned that neighboring gold NPs might fuse together when being brought close to each other during heating since the gold NPs are only stabilized by the end –SH groups of the PNIPAM chains. Similar inter-particle fusion phenomena have also been suggested by others.52,53
Thermal responsive catalytic behavior gold NPs templated by MSPMs
In order to demonstrate the potential of the current templated gold NPs as heterogeneous catalysts, the catalytic activity of the hybrid micelles was investigated by a classic borohydride reduction of p-nitrophenol (p-NP) to p-aminophenol (p-AP).55 It is well established that noble metal nanoparticles can catalyze this reaction and the reaction kinetics can be conveniently monitored by spectroscopic measurements.31 In the p-NP solution under neutral or acidic conditions, a strong absorption peak due to p-NP occurs at 317 nm. The peak shifts to 400 nm due to 4-nitrophenolate ions which become dominant when NaBH4 is added to increase the alkalinity of the solution. The polymeric micelles decorated with gold NPs were then added to the system and the adsorption spectrum was monitored. The peak at 400 nm decreases and approaches a constant value after 8 min, while a new peak at 300 nm appears due to the formation of p-AP (Fig. 5A and S4 in ESI†). There exists a clear isosbestic point between the two absorption bands in the UV spectra. Therefore, the current hybrid micelles have excellent catalytic properties. This is normally attributed to the decreased size of the gold NPs which corresponds to an increased specific area available to the reduction reaction. It is noted here that our system can retain colloidal stability under the high ionic strength due to the reduction agent of NaBH4, which is sometimes problematic for other supported gold NP systems.The kinetics of reduction was determined by measuring the concentration of p-nitrophenolate by recording the absorbance at 400 nm (Fig. 5B). The changes of absorbance with time are straight lines, indicating that the reduction reaction follows first-order kinetics (Fig. 5B). The apparent rate constant (kapp) of the catalytic reactions at 25 °C was estimated to be 5.38 × 10−3 s−1 for MSMP@AuNPs with a PEG/PNIPAM mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, while 1.70 × 10−3 s−1 was estimated for the pure gold NPs. The three times higher efficiency of the former than the latter can be ascribed to the increased specific area of gold NPs at the outmost surface of the micelles. To investigate the influence of temperature on the catalytic behavior of the hybrid micelles, the kinetics of the reduction of p-NP catalyzed by MSPM@AuNPs at 40 °C was also determined, resulting in an kapp of 1.70 × 10−3 s−1 which is nearly identical to that of the pure gold NPs (Fig. 5B). As shown in Fig. 3A, the gold NPs of MSMP@AuNPs turn into solid-like particles with rugged surfaces due to the collapse of the PNIPAM chains upon heating. Therefore the whole particle may act like a pure gold NP.
9, while 1.70 × 10−3 s−1 was estimated for the pure gold NPs. The three times higher efficiency of the former than the latter can be ascribed to the increased specific area of gold NPs at the outmost surface of the micelles. To investigate the influence of temperature on the catalytic behavior of the hybrid micelles, the kinetics of the reduction of p-NP catalyzed by MSPM@AuNPs at 40 °C was also determined, resulting in an kapp of 1.70 × 10−3 s−1 which is nearly identical to that of the pure gold NPs (Fig. 5B). As shown in Fig. 3A, the gold NPs of MSMP@AuNPs turn into solid-like particles with rugged surfaces due to the collapse of the PNIPAM chains upon heating. Therefore the whole particle may act like a pure gold NP.
 | ||
| Fig. 5 Catalytic behavior of gold NPs templated by MSPMs. (A) UV-vis absorption spectra during the reduction of p-NP catalyzed by MSPM@AuNPs. (B) Plot of ln(Ct/C0) versus time during the reduction of p-NP catalyzed by either the citrate acid stabilized gold NPs or MSPM@AuNPs at varied temperature. The MSPM@AuNPs are the same hybrid particles as those in Fig. 3. | ||
Conclusions
In summary, a strategy has been devised to prepare hybrid gold/polymeric NPs using mixed shell polymeric micelles (MSPMs) as the template through the in situ reduction of a gold precursor. Co-assembly of poly(ε-caprolactone)-b-PEG (PCL-b-PEG) and PCL-b-PNIPAM resulted in MSPMs with a PLC core and a mixed PEG/PNIPAM shell. Thiol groups, which are naturally derived from the reduction of the thiocarbonylthio group of the macro chain transfer agent for the reversible addition fragmentation chain transfer polymerization (RAFT), are introduced to the end of the PNIPAM chains. The in situ reduction of the gold precursor in the presence of such MSPMs produce well-dispersed hybrid particles with their outmost surface decorated with gold NPs, which are stabilized by the thiol groups at the end of the PNIPAM chains. The density of the surface thiol groups and therefore the number density of the gold NPs can be tuned by varying the relative mass ratio of the two block polymers, resulting in hybrid particles with different surface plasmon resonance (SPR) properties. Furthermore, the interparticle distance of the gold NPs can be fine-tuned by heating and cooling to induce the shrinking/extension of the PNIPAM chains, resulting in tunable SPR and catalytic properties. The whole hybrid particles are stabilized by the stretched PEG chains in the micellar shell, endowing the current system with excellent colloidal stability under working conditions.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21274067, 91127045, 51390483, 51403093), the Fundamental Research Funds for the Central Universities, the Natural Science Foundation of Tianjin, China (no. 12JCQNJC01800) and PCSIRT (IRT1257).Notes and references
- J. N. Schrauben, R. Hayoun, C. N. Valdez, M. Braten, L. Fridley and J. M. Mayer, Science, 2012, 336, 1298–1301 CrossRef CAS PubMed.
- H. A. Atwater and A. Polman, Nat. Mater., 2010, 9, 205–213 CrossRef CAS PubMed.
- S. Eustis and M. A. El-Sayed, Chem. Soc. Rev., 2006, 35, 209–217 RSC.
- O. V. Salata, J. Nanobiotechnol., 2004, 2, 3 CrossRef PubMed.
- H. Otsuka, Y. Nagasaki and K. Kataoka, Adv. Drug Delivery Rev., 2012, 64, 246–255 CrossRef PubMed.
- F. Kretschmer, S. Mühlig, S. Hoeppener, A. Winter, M. D. Hager, C. Rockstuhl, T. Pertsch and U. S. Schubert, Part. Part. Syst. Charact., 2014, 31, 721–744 CrossRef PubMed.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS.
- J. P. Rao and K. E. Geckeler, Prog. Polym. Sci., 2011, 36, 887–913 CrossRef CAS PubMed.
- M. Elsabahy and K. L. Wooley, Chem. Soc. Rev., 2012, 41, 2545–2561 RSC.
- N. Rapoport, Prog. Polym. Sci., 2007, 32, 962–990 CrossRef CAS PubMed.
- H. Cabral, Y. Matsumoto, K. Mizuno, Q. Chen, M. Murakami, M. Kimura, Y. Terada, M. Kano, K. Miyazono and M. Uesaka, Nat. Nanotechnol., 2011, 6, 815–823 CrossRef CAS PubMed.
- Y. Mai and A. Eisenberg, Chem. Soc. Rev., 2012, 41, 5969–5985 RSC.
- H. Qiu, Z. M. Hudson, M. A. Winnik and I. Manners, Science, 2015, 347, 1329–1332 CrossRef CAS PubMed.
- Z. Zhang, R. Ma and L. Shi, Acc. Chem. Res., 2014, 47, 1426–1437 CrossRef CAS PubMed.
- B. M. Quinn, C. Dekker and S. G. Lemay, J. Am. Chem. Soc., 2005, 127, 6146–6147 CrossRef CAS PubMed.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- T. Wu, Q. Zhang, J. Hu, G. Zhang and S. Liu, J. Mater. Chem., 2012, 22, 5155–5163 RSC.
- H. Xu, J. Xu, X. Jiang, Z. Zhu, J. Rao, J. Yin, T. Wu, H. Liu and S. Liu, Chem. Mater., 2007, 19, 2489–2494 CrossRef CAS.
- J. Ou, C. Chang, Y. Sung, K. Ou, C. Tseng, H. Ling and M. Ger, Colloids Surf., A, 2007, 305, 36–41 CrossRef CAS PubMed.
- J. He, Y. Liu, T. Babu, Z. Wei and Z. Nie, J. Am. Chem. Soc., 2012, 134, 11342–11345 CrossRef CAS PubMed.
- J. Liu, S. Fu, B. Yuan, Y. Li and Z. Deng, J. Am. Chem. Soc., 2010, 132, 7279–7281 CrossRef CAS PubMed.
- X. Lian, J. Jin, J. Tian and H. Zhao, ACS Appl. Mater. Interfaces, 2010, 2, 2261–2268 CAS.
- R. Liang, J. Xu, W. Li, Y. Liao, K. Wang, J. You, J. Zhu and W. Jiang, Macromolecules, 2015, 48, 256–263 CrossRef CAS.
- T. Matsushita, Y. Fukumoto, T. Kawakami, T. Tsuruoka, T. Murashima, T. Yanagishita, H. Masuda, H. Nawafune and K. Akamatsu, RSC Adv., 2013, 3, 16243–16246 RSC.
- J. Han, Y. Liu and R. Guo, Adv. Funct. Mater., 2009, 19, 1112–1117 CrossRef CAS PubMed.
- J. Zhang, S. Xu and E. Kumacheva, J. Am. Chem. Soc., 2004, 126, 7908–7914 CrossRef CAS PubMed.
- M. Karg, I. Pastoriza-Santos, J. Pérez-Juste, T. Hellweg and L. M. Liz-Marzán, Small, 2007, 3, 1222–1229 CrossRef CAS PubMed.
- A. C. Manikas, G. Romeo, A. Papa and P. A. Netti, Langmuir, 2014, 30, 3869–3875 CrossRef CAS PubMed.
- M. Xie, L. Ding, Z. You, D. Gao, G. Yang and H. Han, J. Mater. Chem., 2012, 22, 14108–14118 RSC.
- R. A. McMillan, C. D. Paavola, J. Howard, S. L. Chan, N. J. Zaluzec and J. D. Trent, Nat. Mater., 2002, 1, 247–252 CrossRef CAS PubMed.
- X. Chen, D. Zhao, Y. An, Y. Zhang, J. Cheng, B. Wang and L. Shi, J. Colloid Interface Sci., 2008, 322, 414–420 CrossRef CAS PubMed.
- J. Hu, T. Wu, G. Zhang and S. Liu, J. Am. Chem. Soc., 2012, 134, 7624–7627 CrossRef CAS PubMed.
- J. He, X. Huang, Y.-C. Li, Y. Liu, T. Babu, M. A. Aronova, S. Wang, Z. Lu, X. Chen and Z. Nie, J. Am. Chem. Soc., 2013, 135, 7974–7984 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- J. Du, C. Yu, D. Pan, J. Li, W. Chen, M. Yan, T. Segura and Y. Lu, J. Am. Chem. Soc., 2010, 132, 12780–12781 CrossRef CAS PubMed.
- D. Li, Q. He and J. Li, Adv. Colloid Interface Sci., 2009, 149, 28–38 CrossRef CAS PubMed.
- J. Li, J. Liang, W. Wu, S. Zhang, K. Zhang and H. Zhou, New J. Chem., 2014, 38, 2508–2513 RSC.
- R. Contreras-Cáceres, A. Sánchez-Iglesias, M. Karg, I. Pastoriza-Santos, J. Pérez-Juste, J. Pacifico, T. Hellweg, A. Fernández-Barbero and L. M. Liz-Marzán, Adv. Mater., 2008, 20, 1666–1670 CrossRef PubMed.
- R. A. Álvarez-Puebla, R. Contreras-Cáceres, I. Pastoriza-Santos, J. Pérez-Juste and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2009, 48, 138–143 CrossRef PubMed.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CAS.
- I. Tokarev and S. Minko, Soft Matter, 2012, 8, 5980–5987 RSC.
- H. Lange, B. H. Juárez, A. Carl, M. Richter, N. G. Bastús, H. Weller, C. Thomsen, R. von Klitzing and A. Knorr, Langmuir, 2012, 28, 8862–8866 CrossRef CAS PubMed.
- Y. Wu, F. Zhou, L. Yang and J. Liu, Chem. Commun., 2013, 49, 5025–5027 RSC.
- D. Suzuki and H. Kawaguchi, Langmuir, 2005, 21, 8175–8179 CrossRef CAS PubMed.
- M.-Q. Zhu, L.-Q. Wang, G. J. Exarhos and A. D. Li, J. Am. Chem. Soc., 2004, 126, 2656–2657 CrossRef CAS PubMed.
- X. Liu, Y. Liu, Z. Zhang, F. Huang, Q. Tao, R. Ma, Y. An and L. Shi, Chem.–Eur. J., 2013, 19, 7437–7442 CrossRef CAS PubMed.
- R. Ma, B. Wang, Y. Xu, Y. An, W. Zhang, G. Li and L. Shi, Macromol. Rapid Commun., 2007, 28, 1062–1069 CrossRef CAS PubMed.
- S. De Santis, R. Diana Ladogana, M. Diociaiuti and G. Masci, Macromolecules, 2010, 43, 1992–2001 CrossRef CAS.
- M. Nakayama and T. Okano, Macromolecules, 2008, 41, 504–507 CrossRef CAS.
- A. B. Lowe, B. S. Sumerlin, M. S. Donovan and C. L. McCormick, J. Am. Chem. Soc., 2002, 124, 11562–11563 CrossRef CAS PubMed.
- K. H. Bae, S. H. Choi, S. Y. Park, Y. Lee and T. G. Park, Langmuir, 2006, 22, 6380–6384 CrossRef CAS PubMed.
- G. Wang, J. Lian and T.-Y. Zhang, RSC Adv., 2013, 3, 24017–24020 RSC.
- P. Pengo, L. Pasquato and P. Scrimin, J. Supramol. Chem., 2002, 2, 305–310 CrossRef CAS.
- X. Shen, C. Song, J. Wang, D. Shi, Z. Wang, N. Liu and B. Ding, J. Am. Chem. Soc., 2011, 134, 146–149 CrossRef PubMed.
- N. Pradhan, A. Pal and T. Pal, Langmuir, 2001, 17, 1800–1802 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: XPS, TEM images of gold NP decorated polymeric micelles of PNIPAM or MSPMs of a PEG/PNIPAM mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 after heating, UV-vis absorption spectra during the reduction of 4-NP catalyzed by pure gold NPs or MSPM@AuNPs after heating, See DOI: 10.1039/c5ra06021d 9 after heating, UV-vis absorption spectra during the reduction of 4-NP catalyzed by pure gold NPs or MSPM@AuNPs after heating, See DOI: 10.1039/c5ra06021d |
| This journal is © The Royal Society of Chemistry 2015 |