One-pot synthesis of indolizine via 1,3-dipolar cycloaddition using a sub-equivalent amount of K2Cr2O7 as an efficient oxidant under base free conditions†
Chao Wanga,
Huayou Hu*b,
Juanfang Xuab and
Weiqiu Kanb
aSchool of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, P. R. China
bJiangsu Key Laboratory for Chemistry of Low-Dimensional Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian 223300, P. R. China. E-mail: njuhhy@hotmail.com
First published on 1st May 2015
Abstract
A one-pot method for synthesizing multi-substituted indolizines from α-halo-carbonyl compounds, pyridines and electron deficient alkenes was developed. A sub-equivalent amount of potassium dichromate was used as an oxidant under base free conditions. The transformation developed should be of economic efficiency.
Hydrogenated indolizines, commonly known as indolizidines, are abundant in many natural products and drugs.1 Also indolizines are important heterocycles that exhibit a broad array of bioactivities, for example, antibacterial, phosphatase and aromatase inhibitory, antioxidant, antidepressant and antitumor activities.2 In addition, indolizine derivatives have been extensively applied in analytic chemistry and materials science as fluorescent probes and organic semiconductors respectively.3
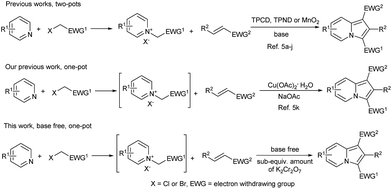
Undoubtedly, methods for synthesizing indolizines are attracting great interests of organic chemists.4,5 Among these reported methods, 1,3-dipolar cycloaddition of electron deficient alkenes with pyridinium ylides in the presence of an oxidant and a base was one of the most efficient because of broad scope and easy availability of starting materials.5 However, several drawbacks were still associated with these methods, such as the use of excess amounts of oxidant and base (Scheme 1).
As regarding to oxidants, oxidants containing Cr(VI) were widely applied in indolizine synthesis, for example tetrakispyridinecobalt(II) dichromate (TPCD)5i and tetrakispyridinenickel(II) dichromate (TPND).5k However, the required use of excessive oxidant and base raised the cost and environmental concerns (Table 1). To circumvent these problems and pursue our interests in indolizines green synthesis,5k,7 we were considered the use of a reagent which could serve as high efficient oxidant and base simultaneously. Under these conditions, the reaction could be base free. Herein, we report the use of sub-equivalent potassium dichromate6 as an oxidant for the one-pot synthesis of indolizines from electron deficient alkenes under base free conditions.
| entry | Oxidant (M. W.a), amount (equiv.)b | Base, amount (equiv.)b | Ref. no. |
|---|---|---|---|
| a M. W. = molecular weight.b Based on the limited starting materials. | |||
| 1 | TPCD (811), 1.0 | Pyridine, 2.5 | 5h |
| 2 | TPND (812), 1.0 | NEt3, 2.2 | 5i |
| 3 | MnO2 (86.9), 8.0 | NEt3, 1.1 | 5j |
| 4 | Cu(OAc)2 (200), 3.0 | NaOAc, 6.0 | 5k |
| 5 | K2Cr2O7 (294), 0.75 | Base free | This work |
In the preliminary study, in order to optimize the reaction condition, we chose 1-(2-oxo-2-phenylethyl)pyridinium bromide 3aa (0.20 mmol, 1.0 equiv.) and butyl acrylate 4a in DMF (N,N-dimethyl formamide, 3.0 mL) as our reaction model system. Several cheap and commercially available Cr(VI) compounds, including potassium dichromate, potassium chromate and chromium trioxide were tested within this system under base free conditions. Fortunately, the desired product 5a was isolated in 68% yield with potassium dichromate as the oxidant. However, using potassium chromate or chromium trioxide, only trace or 36% of 5a were obtained, respectively (Table 2, entry 2 and 3). Furthermore, 0.75 equivalent of potassium dichromate (0.15 mmol) was proven to be most efficient (Table 2, entry 1, 4–6). Further investigation on reaction conditions led us to establish the optimized reaction conditions as follows: 0.75 equivalent of potassium dichromate, 2.0 mL of DMF, heating at 80 °C for 8 h with 1.5 equiv. of 4a (Table 2, entry 10). Notably, the yield of 5aa was just slightly lower when one-pot procedure was applied (Table 1, entry 13).8
| Entry | Oxidant | Amount (mmol) | Ratio (3a/4a) | Solvent (mol L−1) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield.b Other solvents include water, dimethyl sulfoxide, toluene, acetonitrile, 1,4-dioxane and dimethyl carbonate.c One-pot reaction starting from pyridine (1a, 0.21 mmol) and 2-bromo-1-phenylethan-1-one (2a, 0.20 mmol) in 0.20 mL of DMF: the mixture were heated at 60 °C in a test tube with a stopper for 2 h to form 3aa in situ, and then reacted with butyl acrylate (4a, 0.30 mmol) in the presence of potassium dichromate (0.15 mmol) under 80 °C for 8 h with another 1.80 mL of DMF added as solvent. | |||||
| 1 | K2Cr2O7 | 0.13 | 1/2 | DMF (0.067) | 68 |
| 2 | K2CrO4 | 0.26 | 1/2 | DMF (0.067) | Trace |
| 3 | CrO3 | 0.26 | 1/2 | DMF (0.067) | 36 |
| 4 | K2Cr2O7 | 0.15 | 1/1.5 | DMF (0.067) | 95 |
| 5 | K2Cr2O7 | 0.17 | 1/1.5 | DMF (0.067) | 94 |
| 6 | K2Cr2O7 | 0.20 | 1/1.5 | DMF (0.067) | 90 |
| 7 | K2Cr2O7 | 0.15 | 1/1.2 | DMF (0.067) | 93 |
| 8 | K2Cr2O7 | 0.15 | 1/3 | DMF (0.067) | 96 |
| 9 | K2Cr2O7 | 0.15 | 1/1.5 | DMF (0.20) | 78 |
| 10 | K2Cr2O7 | 0.15 | 1/1.5 | DMF (0.10) | 96 |
| 11 | K2Cr2O7 | 0.15 | 1/1.5 | DMF (0.050) | 77 |
| 12 | K2Cr2O7 | 0.15 | 1/1.5 | Other solventsb (0.10) | 0–59 |
| 13c | K2Cr2O7 | 0.15 | 1/1.5 | DMF (0.10) | 93 |
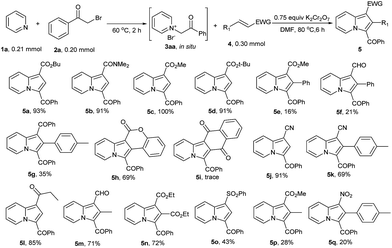
Regarding the substrate scope of alkenes, a series of different electron deficient alkenes reacting with 1a and 2a in a one-pot fashion were studied under standard conditions (Scheme 2). It turned out that most of electron deficient alkenes reacted with 3aa and gave the corresponding product 5 smoothly. However, the derivatives of cinnamic acid, chalcone and (E)-1-methyl-4-(2-nitrovinyl)benzene gave corresponding indolizines (5e–g, 5q) in poor yields. The worst case was naphthalene-1,4-dione, only giving 5i in trace yield. On the contrary, the similar cyclic alkene (coumarin) gave 5h in 69% yield. The reason for the difference is under further investigation.
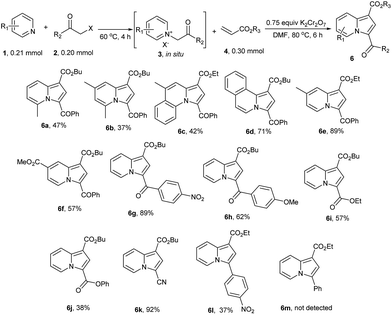
To study the substrate scope of pyridines and α-halide carbonyl compounds, different such substances were reacted with 4a under standard conditions in a one-pot fashion (Scheme 3). As a result, pyridines produced their corresponding products in a smooth manner (Scheme 3, 6a–f). Pyridines with substituents at 2-position gave the corresponding products 6 in moderate yields, possibly due to associated steric hindrance. On the other hand, several α-halo-carbonyl compounds successfully achieved this transformation, including derivatives of α-bromoacetophenone, ethyl 2-bromoacetate, phenyl 2-chloroacetate and 2-bromoacetonitrile (Scheme 3, 6g–k). Interestingly, 6l was obtained in moderate yield (37%) from 1-(bromomethyl)-4-nitrobenzene, but 6m was not detected. Which may due to the low acidity of 1-benzylpyridin-1-ium bromide formed from pyridine and (bromomethyl)benzene.
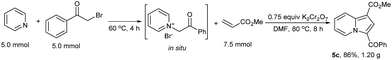
The reaction was performed on a 5.0 mmol scale and producing 5c in 86% yield (1.20 g, Scheme 4), which demonstrated the synthetic utility of this method.
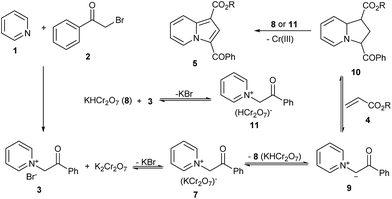
Based on the results of previous study,5 the proposed mechanism of this transformation was presented in Scheme 5. Firstly, pyridinium salt (3) is formed in situ. Then 3 reacts with potassium dichromate to form pyridinium salt 7 with the release of potassium bromide, which would improve the solubility of potassium dichromate in DMF. 9 and 8 are thus generated from 7 by hydrogen abstraction. The dipolar species 9 would readily react with alkene 4 through 1,3-dipolar cycloaddition to form intermediate 10, followed by dehydrogenative aromatization promoted by 8 or 11. Since 8 and 11 contain the same anion with TPCD and TPND which are known to oxidize 10, they are proposed to oxidize 10 to 5 and reduced to Cr(III) species.5 The proposed mechanism is consistent with our experimental result.
Conclusions
A one-pot method for the synthesis of multi-substituted indolizines was developed, starting from α-halo-carbonyl compounds, pyridines and electron deficient alkenes. In this method, sub-equivalent amount of potassium dichromate was used as an oxidant under base free conditions, which rendered this transformation economically efficient. The substrate scopes of this method were broad, since various electron deficient alkenes, pyridines and α-halo-carbonyl compounds were successfully applied in this stage. At last, the synthetic utility has been successfully raised up to a practical gram scale, which may attract the interests of many medicinal and material chemists.Acknowledgements
We are grateful to funding of NSFC (no.: 21202058, 21401063), Education Department of Jiangsu Province (no. 13KJA150001), CPSF (no.: 2012M511645, 2013T60483), MOE (no. 13zs1102) and CSCSE (no. 13zs1102) for their financial supports.Notes and references
- (a) W. Flisch, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984, vol. 4, p. 476 Search PubMed; (b) J. Ewing, G. K. Hughes, E. Ritchie and W. C. Taylor, Nature, 1952, 169, 618 CrossRef CAS.
- (a) E. Reimann, Prog. Chem. Org. Nat. Prod., 2007, 88, 1 CAS; (b) J. P. Michael, Nat. Prod. Rep., 2008, 25, 139 RSC; (c) J. P. Michael, Nat. Prod. Rep., 2003, 20, 458 RSC; (d) J. W. Daly, T. F. Spande and H. M. Garraffo, J. Nat. Prod., 2005, 68, 1556 CrossRef CAS PubMed; (e) M. Lebceuf, A. Cave, A. Ranaivo and H. Moskowitz, Can. J. Chem., 1989, 67, 947 CrossRef CAS; (f) S. A. Snyder, A. M. ElSohly and F. Kontes, Angew. Chem., Int. Ed., 2010, 49, 9693 CrossRef CAS PubMed.
- (a) A. K. Ghosh, G. Bilcer and G. Schiltz, Synthesis, 2001, 2203 CrossRef CAS PubMed; (b) B. List and C. Castello, Synlett, 2001, 1687 CrossRef CAS; (c) P. Sonnet, P. Dallemagne, J. Guillon, C. Enguehard, S. Stiebing, J. Tanguy, R. Bureau, S. Rault, P. Auvray, S. Moslemi, P. Sourdaine and G. E. Seralini, Bioorg. Med. Chem. Lett., 2000, 8, 945 CrossRef CAS; (d) O. B. Ostby, B. Dalhus, L.-L. Gundersen, F. Rise, A. Bast and G. R. M. M. Haenen, Eur. J. Org. Chem., 2000, 3763 CrossRef CAS; (e) B. E. Maryanoff, J. L. Vaught, R. P. Shank, D. F. McComsey, M. J. Costanzo and S. O. Nortey, J. Med. Chem., 1990, 33, 2793 CrossRef CAS; (f) V. H. Lillelund, H. H. Jensen, X. Liang and M. Bols, Chem. Rev., 2002, 102, 515 CrossRef CAS PubMed; (g) J. Gubin, H. D. Vogelaer, H. Inion, C. Houben, J. Lucchetti, J. Mahaux, G. Rosseels, M. Peiren, M. Clinet, P. Polster and P. Chatelain, J. Med. Chem., 1993, 36, 1425 CrossRef CAS; (h) J. Bermudez, C. S. Fake, G. F. Joiner, K. A. Joiner, F. D. King, W. D. Miner and G. J. Sanger, J. Med. Chem., 1990, 33, 1924 CrossRef CAS; (i) A. Vlahovici, M. Andrei and I. Druta, J. Lumin., 2002, 96, 279 CrossRef CAS; (j) A. Vlahovici, I. Druta, M. Andrei, M. Cotlet and R. Dinica, J. Lumin., 1999, 82, 155 CrossRef CAS; (k) T. Mitsumori, M. Bendikov, O. Dautel, F. Wudl, T. Shioya, H. Sato and Y. Sato, J. Am. Chem. Soc., 2004, 126, 16793 CrossRef CAS PubMed; (l) F. D. Saeva and H. R. Luss, J. Org. Chem., 1988, 53, 1804 CrossRef CAS; (m) H. Sonnenschein, G. Henrich, U. Resch-Genger and B. Schulz, Dyes Pigm., 2000, 46, 23 CrossRef CAS; (n) A. V. Retaru, L. D. Druta, T. Deser and T. J. Mueller, Helv. Chim. Acta, 2005, 88, 1798 CrossRef CAS PubMed; (o) F. Delattre, P. Woisel, G. Surpateanu, F. Cazier and P. Blach, Tetrahedron, 2005, 61, 3939 CrossRef CAS PubMed; (p) T. Mitsumori, L. M. Campos, M. A. Garcia-Garibay, F. Wudl, H. Sato and Y. Sato, J. Mater. Chem., 2009, 19, 5826 RSC.
- Selected recent examples for synthesising indolizines: (a) M. F. Z. J. Amaral, A. A. Baumgartner, R. Vessecchi and G. C. Clososki, Org. Lett., 2015, 238 CrossRef CAS PubMed; (b) R. Matczak, B. Koszarna and D. T. Gryko, Tetrahedron, 2014, 70, 7006 CrossRef CAS PubMed; (c) M. Gao, J. Tian and A. Lei, Chem.–Asian J., 2014, 9, 2068 CrossRef CAS PubMed; (d) C. Feng, Y. Yan, Z. Zhang, K. Xu and Z. Wang, Org. Biomol. Chem., 2014, 12, 4837 RSC; (e) B. Koszarna, R. Matczak, M. Krzeszewski, O. Vakuliuk, J. Klajn, M. Tasior, J. T. Nowicki and D. T. Gryko, Tetrahedron, 2014, 70, 225 CrossRef CAS PubMed; (f) L. Xiang, Y. Yang, X. Zhou, X. Liu, X. Li, X. Kang, R. Yan and G. Huang, J. Org. Chem., 2014, 79, 10641 CrossRef CAS PubMed; (g) D. Basavaiah, G. Veeraraghavaiah and S. S. Badsara, Org. Biomol. Chem., 2014, 12, 1551 RSC; (h) X. Wang, S. Li, Y. Pan, H. Wang, H. Liang, Z. Chen and X. Qin, Org. Lett., 2014, 16, 580 CrossRef CAS PubMed; (i) X. Meng, P. Liao, J. Liu and X. Bi, Chem. Commun., 2014, 50, 11837 RSC; (j) X. Tan, Y. Liang, F. Bao, H. Wang and Y. Pan, Tetrahedron, 2014, 70, 6717 CrossRef CAS PubMed; (k) X. Fan, Y. Wang, Y. He, S. Guo and X. Zhang, Eur. J. Org. Chem., 2014, 713 CrossRef CAS PubMed; (l) H. Sun, C. Wang, Y. Yang, P. Chen, Y. Wu, X. Zhang and Y. Huang, J. Org. Chem., 2014, 79, 11863 CrossRef CAS PubMed; (m) S. Mishra, A. K. Bagdi, M. Ghosh, S. Sinha and A. Hajra, RSC Adv., 2014, 4, 6672 RSC; (n) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC; (o) J. H. Lee and I. Kim, J. Org. Chem., 2013, 78, 1283 CrossRef CAS PubMed; (p) M. Li, X. Lv, L. Wen and Z. Hu, Org. Lett., 2013, 15, 1262 CrossRef CAS PubMed; (q) M. Kim, Y. Jung and I. Kim, J. Org. Chem., 2013, 78, 10395 CrossRef CAS PubMed; (r) S. Liu, X. Hu, X. Li and J. Cheng, Synlett, 2013, 24, 847 CrossRef CAS; (s) M. Arisawa, Y. Fujii, H. Kato, H. Fukuda, T. Matsumoto, M. Ito, H. Abe, Y. Ito and S. Shuto, Angew. Chem., Int. Ed., 2013, 52, 1003 CrossRef CAS PubMed; (t) E. J. Choi, E. Kim, Y. Lee, A. Jo and S. B. Park, Angew. Chem., Int. Ed., 2014, 53, 1346 CrossRef CAS PubMed.
- (a) K. M. Dawood, E. A. Ragab and N. A. Khedr, J. Chin. Chem. Soc., 2009, 56, 1180 CrossRef CAS PubMed; (b) E. S. Darwish, Molecules, 2008, 13, 1066 CrossRef CAS PubMed; (c) N. A. Kheder, E. S. Darwish and K. M. Dawood, Heterocycles, 2009, 78, 177 CrossRef CAS PubMed; (d) Y.-M. Shen, B.-X. Wang, Y.-Y. Feng, Z.-Y. Shen, J. Shen, C. Li and H.-W. Hu, Gaodeng Xuexiao Huaxue Xuebao, 2006, 27, 651 CAS; (e) A. Tanaka and T. Usui, Chem. Pharm. Bull., 1979, 27, 3078 CrossRef CAS; (f) B.-X. Wang and H.-W. Hu, Gaodeng Xuexiao Huaxue Xuebao, 2004, 25, 273 CAS; (g) Y. Tominaga, S. Hidaki, Y. Matsuda, G. Kobayashi and K. Sakemi, Yakugaku Zasshi, 1979, 99, 540 CAS; (h) B. Wang, X. Zhang, J. Li, X. Jiang, Y. Hu and H. Hu, J. Chem. Soc., Perkin Trans. 1, 1999, 1571 RSC; (i) B. Wang, T. He, C. Li and H. Hu, Chin. J. Org. Chem., 2003, 23, 794 CAS; (j) L. Zhang, F. Liang, L. Sun, Y. Hu and H. Hu, Synthesis, 2000, 1733 CrossRef CAS PubMed; (k) H. Hu, J. Feng, Y. Zhu, N. Gu and Y. Kan, RSC Adv., 2012, 2, 8637 RSC.
- F. Freeman, Potassium dichromate in e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001 Search PubMed.
- (a) Y. Liu, H. Hu, Q. Liu, H. Hu and J. Xu, Tetrahedron, 2007, 63, 2024 CrossRef CAS PubMed; (b) Y. Liu, H. Hu, Y. Zhang, H. Hu and J. Xu, Org. Biomol. Chem., 2010, 4921 RSC; (c) H. Hu, K. Shi, R. Hou, Z. Zhang, Y. Zhu and J. Zhou, Synthesis, 2010, 4007 CrossRef CAS PubMed; (d) H. Hu, Y. Liu, H. Zhong, Y. Zhu, C. Wang and M. Ji, Chem.–Asian J., 2012, 7, 884 CrossRef CAS PubMed; (e) Y. Liu, H. Hu, X. Su, J. Sun, C. Cao and Y. Shi, Eur. J. Org. Chem., 2013, 2020 CrossRef CAS PubMed; (f) G. Li, H. Hu, Y. Kan and K. Ma, Chin. J. Org. Chem., 2014, 34, 903 CrossRef CAS; (g) H. Hu, Y. Liu, J. Xu, Y. Kan, C. Wang and M. Ji, RSC Adv., 2014, 4, 24389 RSC; (h) J. Sun, H. Hu, F. Wang, H. Wu and Y. Liu, RSC Adv., 2014, 4, 36498 RSC; (i) J. Sun, F. Wang, H. Hu, X. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 3992 CrossRef CAS PubMed; (j) F. Wang, Y. Shen, H. Hu, X. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 9556 CrossRef CAS PubMed; (k) H. Hu, G. Li, W. Hu, Y. Liu, X. Wang, Y. Kan and M. Ji, Org. Lett., 2015, 17, 1114 CrossRef CAS PubMed.
- Experimental procedure for synthesize indolizines: pyridine derivative (1, 0.21 mmol) and α-halide carbonyl compound (2, 0.20 mmol) in 0.20 mL of DMF were heated at 60 °C in a test tube with a stopper for 2 h to form 3 in situ. Then alkene (4, 0.30 mmol), potassium dichromate (0.15 mmol) and another 1.80 mL of DMF were added to the tube. Then the mixture was heated at 80 °C for another 8 h (the reaction course was monitored by TLC). Then the mixture was cooled to r.t., passed through a layer of silica gel and washed with ethyl acetate to remove the inorganic by-products. Then solvent was removed by reduce pressure. The residue was purified by flash chromatogram on silica gel using petroleum ether/ethyl acetate as eluate to give the corresponding indolizine.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data for all new compounds. See DOI: 10.1039/c5ra06019b |
| This journal is © The Royal Society of Chemistry 2015 |