Manganese-phosphomolybdate molecular catalysts for the electron transfer reaction of ferricyanide to ferrocyanide†
Abstract
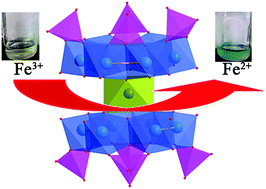
Three manganese-containing phosphomolybdate hybrids (H2bpp)5[Na(Hbpp)]6H10{Mn[Mo6O12(OH)3(HPO4)4]2}4·14H2O (1), [Na4(H2bpp)2Mn(H2O)7]{Mn[Mo6O12(OH)3(HPO4)3(PO4)]2}·2H2O (2), and Na(H2O)2(Hbpp)3[Na2(bpp)(H2O)][Mn2(H2O)5]{Mn[Mo12O24(OH)6(HPO4)6(H2PO4)(PO4)]}·(HPO4)·H2O (3) (bpp = 1,3-bi(4-pyridyl)-propane) have been constructed and characterized. The inorganic moieties of the three hybrids consist of ‘hourglass-shaped’ anionic clusters, composed of two reduced polymolybdenum phosphate units [P4VMo6VO28(OH)3]9 {P4Mo6} bridged by one manganese ion. Preliminary experiments show that these hybrids, as a unique class of molecular catalyst, are highly active for promoting the inorganic electron transfer (redox) reaction of ferricyanide to ferrocyanide by thiosulphate with high rate constants under mild conditions. These catalysts maintain their structural identity both in solution and solid state and can be easily separated from the reaction solution for the next catalytic cycle.

 Please wait while we load your content...
Please wait while we load your content...