A biosensor based on a film bulk acoustic resonator and biotin–avidin system for the detection of the epithelial tumor marker mucin 1
Peng Guoa,
Juan Xiong*a,
Dan Zhenga,
Weihai Zhanga,
Lei Liua,
Shengfu Wangb and
Haoshuang Gu*a
aHubei, Collaborative Innovation Center for Advanced Organic Chemical Materials, Faculty of Physics & Electronic Science, Hubei University, Wuhan 430062, China. E-mail: xiongjuana@163.com; guhsh@hubu.edu.cn
bFaculty of Chemistry and Chemical Engineering, Hubei University, Wuhan 430062, China
First published on 30th July 2015
Abstract
AlN thin film bulk acoustic resonators (FBARs) with a resonant frequency of ∼575 MHz have been fabricated to function as an epithelial tumor marker mucin 1 (MUC1) biosensor. Streptavidin was assembled on the sensitive area of FBAR. After the recognition between aptamers–AuNP conjugates and MUC1, biotin, along with the conjugates, was captured by streptavidin onto the surface of FBARs. Therefore, the target MUC1 could be sensitively detected. This biosensor exhibited a good linear relationship between the frequency shifts and the concentrations of MUC1 ranging from 30 to 500 nM, which indicated the sensitivity is about 818.6 Hz nM−1. The frequency shift remained relatively stable when the concentration of MUC1 was greater than 500 nM since the binding between MUC1 and aptamers–AuNP conjugates reached saturation. The selectivity experiment demonstrated that this biosensor can precisely detect MUC1 with good specificity. The positive results suggest that FBAR is an attractive alternative to a new approach for the detection of MUC1.
1. Introduction
Biosensors have been widely employed as an important tool for biology and biochemical applications where precise and selective detection of target molecules is required.1–3 High sensitivity, label-free, real-time response and continuing miniaturization are the key drivers for the wide spread usage.4,5 The integrated circuit technology has proven to be able to simultaneously mass fabricate the transducers on a single wafer and miniaturize the devices. Therefore, the thin film technology in general has raised increasing interest for new sensors and sensor applications.6,7 Specifically the thin film bulk acoustic resonator (FBAR) technology, in which the propagation of acoustic wave confined at electrode-piezoelectric film-electrode sandwiched structure is changed in response to the analyte captured at the surface, has shown tremendous promise in its versatility in the binding of a variety of label-free biological analytes, owing to the capability of tuning the device surface chemistry.8 Over the past few years, FBAR sensors have been used as mass sensors for biological applications. Lee et al. demonstrated that FBAR can detect the carcinoembryonic antigens which had been widely used as tumor markers.9 Xu et al. had developed the shear mode FBAR biosensors for the detection of aptamer–thrombin binding pair and for the real-time in situ monitoring of the competitive adsorption/exchange of protein.10 More recently, Chen et al. reported acetylcholinesterase-coated FBAR for detecting the organophosphorus pesticides and Zhao et al. reported the anti-human prostate-specific antigen coated FBAR for the detection of human prostate-specific antigen.11,12 For all the applications above, the typical process is assembling biomolecules sensitive membrane, such as antibodies and enzymes, on the surface of FBAR followed by capturing the target biological molecules through the sensitive membrane.13In recent years, the aptamer-based bioassay has attracted tremendous interests because of its excellent properties.14 Aptamers are single-stranded DNA, RNA, or modified nucleic acids which can be obtained by an vitro selection process called systematic evolution of ligands by exponential enrichment (SELEX).15 Compared with antibodies or enzymes, aptamers possess simple synthesis, easy labelling, high affinity and specificity with various kinds of targets, including proteins, thrombin, drugs and other organic or inorganic molecules.16 Because of these advantages, aptamers would be promising molecular receptors in biosensor applications and disease diagnosis.17
Mucins are a family of high molecular weight, heavily glycosylated protein which are produced by epithelial tissues in most metazoans. Mucin 1 (MUC1) is the major of mucus layer found on most human epithelia and serves to lubricate and protect surfaces against mechanical damage, chemical and biological insult.18 MUC1 is also a well-known tumor marker which will be overexpressed on the majority of human epithelia of different origins including breast, gastric, colorectal, lung, prostate, ovarian, pancreatic, and bladder carcinomas.19,20 The increasing amount of MUC1 in blood makes serum assays for MUC1 potentially useful in tumor detection.21 To date, there are limited reports concerning the aptamer-based MUC1 detection. Pang et al. reported a graphene oxide-based fluorescent aptasensor for the turn-on detection of epithelial tumor marker MUC1 in a wide range of 0.04–10 mM.22 Liu et al. proposed a strategy for the sensitive detection of MUC1 based on electrochemiluminescence resonance energy transfer which was shown to detect MUC1 protein in a linear range from 64.9 to 1036.8 nM.23 However, there has no report on the MUC1 detection using FBAR sensors. And several advantageous features such as its smaller size, higher resonant frequency (hence greater sensitivity), lower power consumption and better compatibility with CMOS technology makes it a promising choice for MUC1 detection when compared to electrochemistry based biosensor systems.
In the work reported here, we developed a novel strategy for the detection of MUC1 based on streptavidin-immobilized FBARs by the recognition between aptamers–AuNP conjugates and MUC1 in virtue of specific binding between biotin and avidin. This biosensor exhibits high sensitivity in a large scope and reliable selectivity to the MUC1.
2. Experimental
2.1. Reagents and materials
All chemicals and solvents were of reagent grade or better. HAuCl4, trisodium citrate, mercaptopropionic acid, Tris(2-carboxyethy)phosphine hydrochloride (TCEP), bovine serum albumin (BSA), myoglobin (MYO), cancer embryo antigen (CEA) were purchased from Sigma-Aldrich. Deoxyadenosine triphosphate (dATP) was purchased from Aladdin Chemistry Co., Ltd (Shanghai, China). Streptavidin was purchased from Promega Corp. MUC1 (from the N terminus to the C terminus: PDTRPAPGSTAPPAHGVTS APDTRPAPGSTAPPAHGVTSA) was purchased as a custom synthetic peptide from GL Biochem Co., Ltd (Shanghai, China). Thiol-labelled MUC1 aptamers (5′–HS–(CH2)6-ACA CGG CAG TTG ATC CTT TGG ATA CCC TGG CGT GT-biotin-3′) were acquired from Sangon Biological Engineering Technology & Services Co., Ltd (Shanghai, China). The supporting electrolyte was 0.1 M phosphate buffer solution (PBS) prepared with Na2HPO4 and KH2PO4. The Tris–HCl buffer used in this experiment consisted of 20 mM Tris–HCl (pH 7.4), 100 mM NaCl and 5 mM MgCl2. Doubly distilled water was used for preparing the solutions.2.2. Apparatus
The microstructure of AuNPs, biomolecules and the sectional view of FBAR were determined by field-emission scanning electron microscopy (SEM, JEOL 7100F). The crystal orientation was determined by X-ray diffraction (XRD, Bruker Advanced D8). The sputtering was deposited using the JGP450 sputtering system. The frequency characteristics of the AlN-based FBARs were measured using the vector network analyzer (Agilent E5071C) and probe station (Cascade EPS 150 RF) after standard short-open-load (SOL) calibration.2.3. FBAR sensors fabrication and characterization
FBAR devices were fabricated on double sided polished silicon wafers. A Bragg reflector consisted of alternating layers of Mo and Ti was sputtered on the silicon wafer by DC magnetron sputtering. The highly c-axis oriented AlN film was then deposited with a thickness about 1 μm by RF reactive magnetron sputtering. Finally, an Au thin film of about 100 nm was employed as the electrode and patterned by photolithography and lift-off techniques. The network analyzer was set up to 1601 measuring points within the frequency sweep. The data obtained were then read out and recorded. The measurements were carried out in an ambient room in which the temperature was kept at 25 °C. The characterizations of fabricated FBARs as shown in Fig. 1 indicate the resonant frequency of the FBAR was typically around 575 MHz. The electromechanical coupling coefficient and the quality factor for the bare FBAR were about 3.74% and 384, respectively.2.4. Fabrication of MUC1 aptamers–AuNP conjugates
Before the preparation, all glassware was thoroughly cleaned in aqua regia (3 parts HCl and 1 part HNO3), rinsed in doubly distilled water, and oven-dried prior to use. First, 1 mL of AuNPs solution, 50 μL of MUC1 aptamers buffer (10 μM aptamers dissolved in Tris–HCl buffer) and 6 μL of Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) were successively added into the glass bottle followed by shaking for 2 h. In this step, AuNPs solution was prepared in advance using the HAuCl4 solution and trisodium citrate. The MUC1 aptamer was modified with thiol at the 5′-end and biotin at the 3′-end. TCEP, as one kind of effective reducing agents for thiol, was used to cut off disulfide bond. Secondly, 100 μL of 14.1 μM deoxyadenosine triphosphate (dATP) was added to the solution and shaken for an hour. And then the solution was kept for 3 h at room temperature to increase the stability of aptamers–AuNP conjugates. At last the conjugates were centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, followed by the removal of the supernatant. The precipitate was then dispersed in 0.01 M PBSB (PBS pH 7.4, 1% BSA), and stored at 4 °C for further use.
000 rpm for 10 min, followed by the removal of the supernatant. The precipitate was then dispersed in 0.01 M PBSB (PBS pH 7.4, 1% BSA), and stored at 4 °C for further use.
2.5. Assembling streptavidin onto FBAR
At first, the FBAR resonator was sonicated with ethanol and double-distilled water for 10 min under the low power to remove the impurities on the surface of FBAR, followed by N2 drying at room temperature. And then, 3μL of mercaptopropionic acid (2 mM) was added to the resonance zone and kept for an hour. Then the resonator was washed by PBS solution to remove the impurities and dried in air for 2 h. 3 μL of carbodiimide/N-hydroxysuccinimide (EDC/NHS; 400 mM per 100 mM) was added to the resonance zone and kept for an hour. Like before, the resonator was washed by PBS solution to remove the impurities and dried at room temperature. 3 μL of streptavidin (2 mg mL−1) was then added to the resonance zone and kept for another 4 h. At last, the resonator was washed by PBS solution and double-distilled water, and then dried at room temperature for the detection of resonant frequency.2.6. Procedures of MUC1 detection
For a typical MUC1 detection experiment, 10 μL MUC1 solution of different concentrations (30–1000 nM) was introduced in the solution of 10 μL MUC1 aptamers–AuNP conjugates and followed by incubated at 37 °C for 2 h to finish the recognition between the MUC1 and MUC1 aptamers. Then, 2 μL of the mixed solution was dropped on the surface of streptavidin assembled FBAR and incubated for 1 h. Measurements were then performed after buffer wash, gentle nitrogen drying. The selectivity of the immobilized sensor was also determined. For the selectivity experiments, frequency shifts were monitored with the similar procedure as the typical MUC1 detection experiments with introduction of BSA, MYO and CEA solutions at 500 nM in the solution of MUC1 aptamers–AuNP conjugates.3. Results and discussion
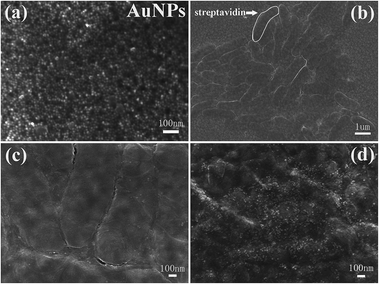
In this work, biotin and avidin system was used with FBARs for the detection of the epithelial tumor marker MUC1. And the specific reaction between activated biotin and pre-immobilized streptavidin could realize 4-fold signal amplification because every streptavidin could bind four biotins.24 Before the detection of MUC1, the MUC1 aptamers were immobilized on AuNPs by self-assembly via Au–S bonds. As illustrated in Fig. 2, the MUC1 aptamers which have thiol on the end are firstly immobilized on AuNPs by self-assembly via Au–S bonds to yield aptamers–AuNP conjugates. In the absence of target MUC1, the immobilized MUC1 aptamer is in a “closed” state, which shields biotin from being captured by the streptavidin.18 As a result, the aptamers–AuNP conjugates cannot be captured by the streptavidin-modified electrode (Fig. 2(a)). In the presence of MUC1, the MUC1 aptamer is disrupted, after that the biotin is exposed. Then the biotin, along with the dually labelled conjugates, is then easily captured by the streptavidin-modified electrode (Fig. 2(b)). In virtue of the mass-loading sensitivity of FBAR, the resonant frequency would decrease after aptamers–AuNP conjugates loading. Therefore, the detection of target MUC1 can be transduced via detection of the resonant frequency shifts in a round-out way.We characterized the morphologies by SEM. As shown in Fig. 3(a), the image of AuNPs prepared by HAuCl4 solution and trisodium citrate indicates that AuNPs have a diameter of approximately 15–20 nm and a narrow size distribution. Fig. 3(b) shows the top view of streptavidin-assembled Au electrode surface of FBAR. It can be found that the streptavidin exhibits uniform distribution onto the surface of FBAR. As for control experiment, only a little amount of AuNPs can be found on the surface of the streptavidin-immobilized FBAR without binding with MUC1, as shown in Fig. 3(c). After MUC1 aptamers–AuNP conjugates combined with 500 nM MUC1, a large number of uniform AuNPs can be clearly observed on the surface of streptavidin-immobilized FBAR, which demonstrates the target molecule MUC1 has promoted aptamers–AuNP conjugates binding to the surface of streptavidin-immobilized FBAR, as shown in Fig. 3(d).
In order to verify the experimental results shown in Fig. 3, the return loss (S11) parameters of FBARs were measured before and after streptavidin adsorption, and after aptamers–AuNP conjugates immobilization on FBAR with and without MUC1. Fig. 4 demonstrates the resonant frequency drifting down by about 450 kHz of the two FBARs after streptavidin adsorption, respectively, due to the mass loading effect. However, no further noticeable frequency shift is detected after binding AuNPs without MUC1, as can be seen in Fig. 4(a). Because the MUC1 aptamer immobilized on AuNPs is in a “closed” state with the absence of MUC1, the recognition between biotin and streptavidin is therefore ignorable. So, the resonant frequency shift is negligible. While a distinct frequency drop about 438 kHz is observed after binding AuNPs along with 500 nM target MUC1 in Fig. 4(b). In the presence of MUC1, the MUC1 aptamer is disrupted resulting in the exposure of biotin. The recognition between biotin and streptavidin is then fluent, so the resonant frequency shift is obvious. The result of this control experiment clearly indicated that streptavidin assembled FBAR has no response without target MUC1and can be used to detect MUC1.
Fig. 5 plots the frequency shifts of the streptavidin-immobilized FBAR sensors after binding aptamers–AuNP conjugates along with various concentrations of target MUC1 ranging from 30–1000 nM. A linear relationship between the resonant frequency shifts and the MUC1 concentrations in the range of 30–500 nM can be observed. However, the frequency shifts kept relatively stable when the concentration of MUC1 was greater than 500 nM. In our experiment, the volume ratio of target MUC1 to the aptamers–AuNP conjugates was set to 1 and the concentration of aptamers–AuNP conjugates was about 500 nM as designed. The binding between MUC1 and aptamers–AuNP conjugates reached a maximum value when the concentration of MUC1 was 500 nM. The unconjugated target MUC1 had been rinsed from the surface of FBAR sensor, and therefore no continuous frequency shift can be found in Fig. 5. The sensitivity of this MUC1 FBAR biosensor is 818.6 Hz nM−1 which can be obtained by the slope of the calibration curve.
In our work, it is noticeable that the frequency shift is still great although the concentration of MUC1 is as low as 30 nM. We attribute two key components to the great frequency shift. Each streptavidin could bind four biotins, which indicated 4-fold signal amplification was achieved by the specific reaction between active biotin and the immobilized streptavidin. Large amount of AuNPs were coated on the streptavidin-immobilized surface of FBAR sensor simultaneously when the biotins were captured by streptavidin. And the additional loading of AuNPs would also contribute to the increasing frequency shift of FBAR sensor. Therefore, the sensor combined FBAR and biotin–avidin system for the detection of MUC1 exhibits a prominent sensitivity.
A control experiment of non-specific bonding of MUC1 with aptamers–AuNP conjugates on streptavidin-immobilized FBARs was also carried out. And the data are shown in Fig. 6. The streptavidin-immobilized FBARs were used to detect BSA, MYO, CEA, and MUC1, some of which belong to the tumor mark family. Adsorption of aptamers–AuNP conjugates along with MUC1 (500 nM) on the streptavidin immobilized FBAR resulted in a frequency shift down of 451 kHz, which demonstrated a higher sensitivity to target MUC1. The introduction of BSA, MYO, and CEA on streptavidin-immobilized FBARs only resulted in about 30 kHz frequency shift indicating the negligible non-specific binding. These results suggested that the FBAR biosensor was able to discriminate MUC1 from other tumor markers.
 | ||
| Fig. 6 The frequency shifts of streptavidin-immobilized FBARs after binding with MUC1, BSA, MYO and CEA at 500 nM. Error bars are obtained based on three independent measurements. | ||
4. Conclusions
In this study, we have fabricated film bulk acoustic resonator biosensor for detection of epithelial tumour marker MUC1 based on the aptamers–AuNP conjugates. The sensitive detection was achieved by the specific bonding between biotin and streptavidin. The performance of the FBAR sensor for various MUC1 concentrations ranging from 30 to 1000 nM was thoroughly investigated. This biosensor exhibited good linear relationship between the frequency shifts and the concentrations of MUC1 over the range of 30–500 nM, which indicated the sensitivity of this biosensor was about 818.6 Hz nM−1. The frequency shift kept relatively stable when the concentration of MUC1 was greater than 500 nM since the binding between MUC1 and aptamers–AuNP conjugates reached a maximum value. Subsequent selectivity experiment demonstrated the biosensor could precisely detect MUC1 with good specificity. Therefore, we can draw a conclusion that the FBAR sensor provides a new approach for the detection of MUC1. It also shows a promising future for point-of-care diagnosis of genetic diseases and for the detection of cancer.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 61106070), the Applied Basic Research Programs of Wuhan city (no. 2014010101010006), and the Key Project of Natural Science Foundation of Hubei Province of China (Grand no. 2013CFA043).Notes and references
- Q. Liu, C. Wu, H. Cai, N. Hu, J. Zhou and P. Wang, Chem. Rev., 2014, 114, 6423–6461 CrossRef CAS PubMed.
- L. García-Gancedo, Z. Zhu, E. Iborra, M. Clement, J. Olivares, A. J. Flewitt, W. I. Milne, G. M. Ashley, J. K. Luo, X. B. Zhao and J. R. Lu, Sens. Actuators, B, 2011, 160, 1386–1393 CrossRef PubMed.
- U. Mastromatteo and F. F. Villa, Sens. Actuators, B, 2013, 179, 319–327 CrossRef CAS PubMed.
- J. Weber, W. M. Albers, J. Tuppurainen, M. Link, R. Gabl, W. Wersing and M. Schreiter, Sens. Actuators, A, 2006, 128, 84–88 CrossRef CAS PubMed.
- X. Yang and E. Wang, Anal. Chem., 2011, 83, 5005–5011 CrossRef CAS PubMed.
- F. Xue, L. Zhang, W. Tang, C. Zhang, W. Du and Z. L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5955–5961 CAS.
- N. Enkin, E. Sharon, E. Golub and I. Willner, Nano Lett., 2014, 14, 4918–4922 CrossRef CAS PubMed.
- G. Wingqvist, Surf. Coat. Technol., 2010, 205, 1279–1286 CrossRef CAS PubMed.
- T. Y. Lee and J. T. Song, Thin Solid Films, 2010, 518, 6630–6633 CrossRef CAS PubMed.
- W. Xu, S. Choi and J. Chae, Appl. Phys. Lett., 2010, 96, 053703 CrossRef PubMed.
- D. Chen, J. Wang, Y. Xu and D. Li, Sens. Actuators, B, 2012, 171–172, 1081–1086 CrossRef CAS PubMed.
- X. Zhao, F. Pan, G. M. Ashley, L. Garcia-Gancedo, J. Luo, A. J. Flewitt, W. I. Milne and J. R. Lu, Sens. Actuators, B, 2014, 190, 946–953 CrossRef CAS PubMed.
- E. Kim, Y.-K. Choi, J. Song and J. Lee, Mater. Res. Bull., 2013, 48, 5076–5079 CrossRef CAS PubMed.
- A. Chen and S. Yang, Biosens. Bioelectron., 2015, 71, 230–242 CrossRef CAS PubMed.
- L. Cai, Z. Z. Chen, M. Y. Chen, H. W. Tang and D. W. Pang, Biomaterials, 2013, 34, 371–381 CrossRef CAS PubMed.
- Z. Zhang, S. Zhang, S. Liu, M. Wang, G. Fu, L. He, Y. Yang and S. Fang, Sens. Actuators, B, 2015, 220, 184–191 CrossRef CAS PubMed.
- K. L. Rhinehardt, G. Srinivas and R. V. Mohan, J. Phys. Chem. B, 2015, 119, 6571–6583 CrossRef CAS PubMed.
- R. Hu, W. Wen, Q. Wang, H. Xiong, X. Zhang, H. Gu and S. Wang, Biosens. Bioelectron., 2014, 53, 384–389 CrossRef CAS PubMed.
- S. Nath and P. Mukherjee, Trends Mol. Med., 2014, 20, 332–342 CrossRef CAS PubMed.
- J. van den Bossche, W. T. Al-Jamal, B. Tian, A. Nunes, C. Fabbro, A. Bianco, M. Prato and K. Kostarelos, Chem. Commun., 2010, 46, 7379–7381 RSC.
- C. Zhu, G. Yang, H. Li, D. Du and Y. Lin, Anal. Chem., 2015, 87, 230–249 CrossRef CAS PubMed.
- Y. He, Y. Lin, H. Tang and D. Pang, Nanoscale, 2012, 4, 2054–2059 RSC.
- W. Wei, D. F. Li, X. H. Pan and S. Q. Liu, Analyst, 2012, 137, 2101 RSC.
- Y. Pan, W. Shan, H. Fang, M. Guo, Z. Nie, Y. Huang and S. Yao, Biosens. Bioelectron., 2014, 52, 62–68 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |