A systematic understanding of gelation self-assembly: solvophobically assisted supramolecular gelation via conformational reorientation across amide functionality on a hydrophobically modulated dipeptide based ambidextrous gelator, N-n-acyl-(l)Val-X(OBn), (X = 1,ω-amino acid)†
Abstract
A systematic investigation on gelation self-assembly has been performed on a hydrophobically modulated dipeptide based ambidextrous gelator, N-n-acyl-(L)Val-X(OBn), (X = 1,ω-amino acid). To elucidate the effect of hydrophobic tuning on gelator architecture towards its gelation self-assembly, three sets of gelators with a common formula: CmH2m+1C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH(L)Val(C
O)NH(L)Val(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–(CH2)n–(C
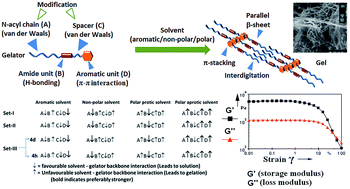
O)NH–(CH2)n–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OBn, were synthesized, Set-I includes gelators with n = 2, m = 9, 11, 13, 15, 17, for Set-II it is n = 2, 3, 5, m = 13 and Set-III comprises of two isomeric gelators (n = 2, m = 15; n = 10, m = 7). Gelation has been critically analyzed in various apolar (aromatic and aliphatic) and polar (protic and aprotic) solvents using FESEM, CD, IR, WAXRD and rheological studies. Obtained results reveal that π–π type interaction dictates the primary molecular alignment and positioning of amide functionality across the aliphatic chain which influences the peptidic orientation in parallel (when m > n) or antiparallel (when m < n) β-sheet type organization in their self-assembly. The thermal stability, gelation number (GN), Tgel and yield stress of gel systems increases with m, but for a given m, the trend goes apparently inverse with the increasing n. Circular dichroism (CD) studies suggest an intriguing evidence of non-planarity of amide plane during self-assembly, highlighting the involvement of conformational change taking place during molecular organization towards its gelation. Despite complex nature of solvent–gelator interaction, the effect of H-bonding component of solubility parameters was found to have a significant role on self-assembly. Overall, supramolecular forces acting at specific functionalities encrypted in gelator backbone must overcome the solvation energy with synergic assistance of solvophobic effect towards stabilization of gel-network with optimum gelator backbone conformation for achieving required enthalpic contribution for self-assembly.
O)OBn, were synthesized, Set-I includes gelators with n = 2, m = 9, 11, 13, 15, 17, for Set-II it is n = 2, 3, 5, m = 13 and Set-III comprises of two isomeric gelators (n = 2, m = 15; n = 10, m = 7). Gelation has been critically analyzed in various apolar (aromatic and aliphatic) and polar (protic and aprotic) solvents using FESEM, CD, IR, WAXRD and rheological studies. Obtained results reveal that π–π type interaction dictates the primary molecular alignment and positioning of amide functionality across the aliphatic chain which influences the peptidic orientation in parallel (when m > n) or antiparallel (when m < n) β-sheet type organization in their self-assembly. The thermal stability, gelation number (GN), Tgel and yield stress of gel systems increases with m, but for a given m, the trend goes apparently inverse with the increasing n. Circular dichroism (CD) studies suggest an intriguing evidence of non-planarity of amide plane during self-assembly, highlighting the involvement of conformational change taking place during molecular organization towards its gelation. Despite complex nature of solvent–gelator interaction, the effect of H-bonding component of solubility parameters was found to have a significant role on self-assembly. Overall, supramolecular forces acting at specific functionalities encrypted in gelator backbone must overcome the solvation energy with synergic assistance of solvophobic effect towards stabilization of gel-network with optimum gelator backbone conformation for achieving required enthalpic contribution for self-assembly.

 Please wait while we load your content...
Please wait while we load your content...