Hybridization of PDMS based cyanate ester and DGEBA for radiation resistant and microelectronics applications
Mathivathanan Ariraman,
Ramachandran Sasikumar and
Muthukaruppan Alagar*
Polymer Composites Lab, Department of Chemical Engineering, A.C.Tech, Anna University, Chennai-600 025, India. E-mail: mkalagar@yahoo.com
First published on 9th July 2015
Abstract
Cyanate ester terminated polydimethylsiloxane (PDMS-OCN) was synthesized and is copolymerized with DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios) through the formation of oxazoline ring to obtain a thermally stable and flexible hybrid PDMS–DGEBA polymer matrix. Subsequently, the radiation resistant behavior of PDMS–DGEBA is studied by a UV treatment process. UV irradiated PDMS–DGEBA films possess an excellent radiation resistance due to the formation of a passive silica layer on the surface of the sample, which effectively protects the materials from UV rays. The silica layer formation was confirmed by scanning electron microscopy (SEM) images. Moreover, X-ray photoelectron spectroscopy (XPS) analysis of PDMS–DGEBA also supports the increasing percentage of silica content after the irradiation of UV rays. Furthermore, the lower values of dielectric constant and dielectric loss, higher thermal stability and excellent radiation resistant properties of PDMS–DGEBA confirm that it can be used as an effective interlayer for low k dielectrics as an insulating material in ultra large scale integrated circuitry (ULSIC) devices.
1 ratios) through the formation of oxazoline ring to obtain a thermally stable and flexible hybrid PDMS–DGEBA polymer matrix. Subsequently, the radiation resistant behavior of PDMS–DGEBA is studied by a UV treatment process. UV irradiated PDMS–DGEBA films possess an excellent radiation resistance due to the formation of a passive silica layer on the surface of the sample, which effectively protects the materials from UV rays. The silica layer formation was confirmed by scanning electron microscopy (SEM) images. Moreover, X-ray photoelectron spectroscopy (XPS) analysis of PDMS–DGEBA also supports the increasing percentage of silica content after the irradiation of UV rays. Furthermore, the lower values of dielectric constant and dielectric loss, higher thermal stability and excellent radiation resistant properties of PDMS–DGEBA confirm that it can be used as an effective interlayer for low k dielectrics as an insulating material in ultra large scale integrated circuitry (ULSIC) devices.
Introduction
Polycyanurate is one of the important classes of thermosetting polymers widely used in the field of microelectronics. These cyanurate polymers meet all industrial requirements such as mechanical strength, thermal stability, metal adhesive property, low moisture uptake and electrical properties.1–5 The low dielectric constant and good mechanical behavior of polycyanurate attracted scientists to develop a new type of skeletally modified cyanate ester from sustainable phenolic resources.5–7 Consequently, eugenol based bisphenols have been synthesized using polydimethyl siloxane (PDMS)8 and are utilized to develop cyanate ester because eugenol is a sustainable phenolic material that can be extracted from cloves. These cloves are the aromatic flower buds of a tree in the family Myrtaceae, Syzygium aromaticum.9 The main drawback of polycyanurate based thermosetting polymers is brittleness, which can be overcome using ether linked long chain materials. Diglycidalether bisphenol-A (DGEBA) has been widely used to increase the flexibility of the polymer composites through the formation of oxazoline ring between DGEBA and cyanate ester.10,11PDMS is an organic–inorganic hybrid long chain polymer material containing dimethylsiloxane units, which significantly contribute to increase the flexibility and hydrophobicity and polarizability of the materials along with improved thermal stability.12 Due to these properties, it can be used to develop composite materials for use in the fields of optical, electrical and micro-fluidic applications.12 Most satellites are being launched into low earth orbit (LEO) altitudes and positioned in the range between 200 km and 1000 km. LEO space area is an aggressive environment because atomic oxygen (AO), ultraviolet (UV) radiation, ionizing radiation, vacuum-ultraviolet (VUV), thermal cycles, micrometeoroids and orbital fragments cause damage to satellite materials.13,14 To overcome these damages, UV shielding materials are warranted. The PDMS based composites have been employed as UV-ozone/UV radiation resistant materials.15–17 Furthermore, according to the ITRS roadmap, a dielectric constant of below 2.1 is needed to reduce the packaging density cross talk, leakage current and power consumption in the ultra large scale integrated circuitry devices.18
Therefore, in the present study, cyanate ester terminated PDMS (PDMS-OCN) has been synthesized using sustainable eugenol material and subsequently the PDMS-OCN was utilized to develop the PDMS–DGEBA polymer composites by the copolymerization of PDMS-OCN with DGEBA through the formation of an oxazoline ring. Then, the dielectric, UV resistant, hydrophobic and thermal properties of prepared PDMS–DGEBA composites have been studied, discussed and reported.
Experimental
Materials
Analytical grade cyanogen bromide (CNBr), triethylamine (TEA), and solvents were purchased from SRL, India. DGEBA, hydrogen terminated PDMS (molecular weight ∼580) and platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex solution in xylene [Pt(dvs)] catalyst were purchased from Javanthee Enterprises Guindy, Chennai, India, and Sigma-Aldrich and were used as received without further purification. Eugenol was purchased from Alfa Aesar, India.Synthesis of PDMS-OH
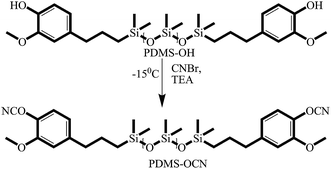
Hydroxyl terminated polydimethylsiloxane (PDMS-OH) has been synthesized through Michael addition reaction between hydride terminated PDMS and eugenol. To a stirred solution of hydride terminated PDMS (8.5 g, 1 mol) in toluene, 3 drops of Pt(dvs) were added under nitrogen atmosphere and stirred for 30 min at 30 °C. Followed by the addition of eugenol (3.89 g, 2.5 mol), the reaction mixture was stirred overnight at 120 °C. After the completion of reaction, the temperature was reduced to 30 °C and activated charcoal was added to the reaction mixture and then filtered through celite. Then, the filtrate was concentrated under reduced pressure using a rotary evaporator to obtain the product (94% yield, brown semi-solid).Synthesis of PDMS-OCN
Cyanate ester terminated PDMS (PDMS-OCN) was synthesized by the following procedure.19 PDMS-OH was dissolved in dry acetone under a nitrogen atmosphere and then the solution of cyanogen bromide in acetone was added to the reaction mixture, followed by the slow addition of triethylamine at −15 °C. Subsequently, the temperature of the reaction mixture was slowly increased to room temperature and stirred for 1 h. Then, the reaction mixture was filtered, the filtrate was concentrated under reduced pressure, and the concentrated product was dissolved in dichloromethane and poured into cold water and extracted with dichloromethane to remove the unreacted materials. Finally, the extracted organic layer was collected and concentrated under reduced pressure, and the product was obtained as a light brown semi-solid (92% yield), which was stored at 0 °C (Scheme 2).Synthesis of PDMS–DGEBA polymer composites
Two different ratios of PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
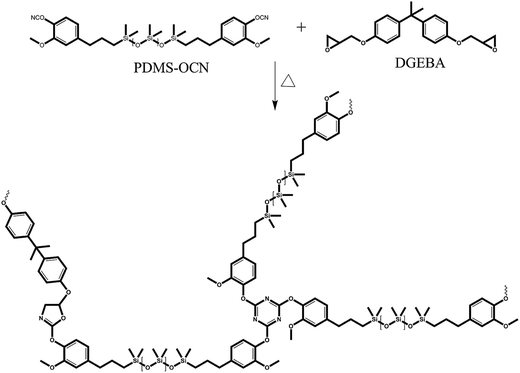
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) polymer composites were prepared through the sol–gel method using PDMS-OCN and DGEBA (Scheme 3). To a clear solution of PDMS-OCN in THF, DGEBA was slowly added and stirred for 15 min at 30 °C. The resulting viscous solution was poured into a respective glass mold and then the solvent was evaporated at 50 °C for 3 h. Furthermore, the temperature was slowly increased to 250 °C at a heating rate of 20 °C h−1. The cured films were then peeled off and directly used for further characterization.
1) polymer composites were prepared through the sol–gel method using PDMS-OCN and DGEBA (Scheme 3). To a clear solution of PDMS-OCN in THF, DGEBA was slowly added and stirred for 15 min at 30 °C. The resulting viscous solution was poured into a respective glass mold and then the solvent was evaporated at 50 °C for 3 h. Furthermore, the temperature was slowly increased to 250 °C at a heating rate of 20 °C h−1. The cured films were then peeled off and directly used for further characterization.
Characterization
FTIR spectroscopy was performed on a Bruker (TENSOR 27) using the KBr pellet method. 1H and 13C NMR spectra were obtained on a Bruker 500 NMR spectrometer. Thermogravimetric measurements were carried out on a Q500 Hi-Res TGA from TA instruments thermogravimetric analyzer. The samples (about 10 mg) were heated from ambient temperature to 700 °C under a continuous flow of nitrogen (20 mL min−1) at a heating rate of 10 °C min−1. X-Ray photoelectron spectroscopy analysis of the samples was carried out using a JEOL JPS-9200 photoelectron spectrometer with a monochromatized Al-Kα X-ray source operated at 12 kV and 20 mA.Scanning electron microscopy (SEM) measurements were performed using a VEGA 3 TESCAN scanning electron microscope. The piece of film was fixed on the surface of a double-sided adhesive tape, and the film was sputtered with gold prior to SEM observation. Dielectric constant was determined by Broadband Dielectric Spectrometer (BDS), NOVOCONTROL Technologies GmbH & Co. (model Concept 80) at 30 °C in the range of 1 Hz to 1 MHz. Optical transparency of the polymeric nanocomposite film was characterized by a Shimadzu UV-2450 UV-visible spectrometer.
Results and discussion
Characterization of PDMS-OH
Hydroxyl terminated PDMS (PDMS-OH) has been synthesized (Scheme 1) and confirmed by FTIR, 1H, 13C and DEPT-135 NMR, as shown in Fig. 1. The 1H NMR spectrum (Fig. 1a) shows peaks in the ranges from 6.75 to 6.56 ppm, 3.81 to 3.75 ppm and at 5.49 ppm, representing the aromatic, Ar-O–CH3, and phenolic hydroxyl proton, respectively. The peaks related to Ar-CH2–C, Si–CH2–C and Si–CH3 protons appear in the regions of 2.49–1.49 ppm, 0.53–0.48 ppm, and 0.10 ppm, respectively. Fig. 1c shows 13C NMR spectrum, the appearance of peaks in the ranges from 145.31 to 110.06 ppm represent aromatic carbon and the aliphatic protons appearing between 54.73 and 0.12 ppm as described in the Experimental part. In addition, DEPT-135 NMR was utilized to confirm the selective addition of allylic double bonds of eugenol to H-PDMS. DEPT-135 NMR only shows the CH and CH3 peaks that appeared in the positive region; the CH2 peaks are in the negative region apart from the C peak.20 From Fig. 1b, it can be clearly observed that the peaks related to three CH2 peaks (Ar-CH2–CH2–CH2–Si) are present in the negative region, confirming the formation of product, as shown in Scheme 1. The 29Si NMR (Fig. 2) spectrum of PDMS-OH also supports the formation of PDMS-OH. The peaks appearing at −18.09 ppm and 11.43 ppm represent Si atoms with two different chemical environments. | ||
| Fig. 1 (a) 1H NMR spectrum of PDMS-OH, (b) DEPT-135 NMR spectrum of PDMS-OH, (c) 13C NMR spectrum of PDMS-OH, and (d) 13C NMR spectrum of PDMS-OCN. | ||
Characterization of PDMS-OCN
PDMS-OH was treated with cyanogen bromide in the presence of base to obtain cyanate ester terminated PDMS (PDMS-OCN), and the formation of PDMS-OCN was confirmed by 13C NMR spectrum and FTIR. Fig. 1d shows the 13C NMR spectrum of PDMS-OCN, a new carbon peak related to OCN appeared at 108.66 ppm, indicating the formation of PDMS-OCN. Fig. 3a and b shows FTIR spectra of PDMS-OCN and PDMS–DGEBA polymer matrix. The appearance of band (Fig. 3a) at 2342 cm−1 represents –OCN and the bands corresponding to Si–O–Si and aliphatic CH stretching frequencies appear at 1067 cm−1 and 2954 cm−1, respectively. After the copolymerization of PDMS-OCN and DGEBA, the FTIR spectrum (Fig. 3b) of PDMS–DGEBA shows the disappearance of the band related to –OCN at 2342 cm−1 and the appearance of new bands at 1270 cm−1 and 1588 cm−1, which are attributed to the formation of triazine rings (trimerization of cyanate ester) and oxazoline rings (reactions of epoxy with cyanate ester), respectively.5 In addition, the band at 1738 cm−1 represents the conversion of the triazine ring into isocyanurate, which is observed at a higher temperature.10 From the FTIR spectral analysis, it can be clearly inferred that the polymer matrices formed through the formation of triazine and oxazoline rings.Surface morphology
The surface properties of PDMS–DGEBA have been examined using scanning electron microscopy (SEM), as shown in Fig. 4. The prepared PDMS–DGEBA films show smooth surfaces (Fig. 4a and b), whereas the films after UV irradiation at 365 nm light source for the period of one week (168 h) show (Fig. 4c and d) the aggregation of silica particles that are linked together and form silica covered surfaces. Consequently, the PDMS–DGEBA coated materials were protected from UV rays by the formation of a silica passive layer. Moreover, in the surface of the films, the formation of silica passive layers effectively protects the surface from UV radiation. In addition, Scheme 4 represents the tentative mechanism of the formation of silica passive layer on the surface of polymer.21–23 Moreover, during the UV-irradiation, the surface of the polymer undergoes oxidation or scission that occurs primarily at the ester linkage in the polycyanurate and siloxane network resulting in the generation of nitrogen containing triazine rings and silica passive layers.23 Furthermore, Owen and Smith suggested that a SiOx layer is believed to form when pure siloxane-type systems are treated with UV-irradiation.24 This was further supported by XPS analysis of PDMS/DGEBA polymer sample before and after UV-irradiation, which clearly ascertains the variation in the concentration of such Si, C and O atoms present in the polymer network. | ||
| Fig. 4 SEM images of before UV irradiation of PDMS–DGEBA (a and b) and after UV irradiation of PDMS–DGEBA (c and d). | ||
Structural analysis
X-Ray photoelectron spectroscopy (XPS) analysis was utilized to confirm the composition and the presence of different types of chemical bonding in the matrix. The elements present in the polymer matrix have been confirmed using XPS survey spectrum (Fig. 5). Thus, the PDMS–DGEBA matrix before and after UV irradiation has been analyzed by XPS. Fig. 5a and b show the survey spectra of PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) matrix before (Fig. 5a) and after (Fig. 5b) UV exposure. After UV irradiation, the structural changes and silica layer formation on the surface of the PDMS–DGEBA polymer matrix can be confirmed by the intensity of C1s, O1s and Si2p atomic percentages. The increase in the intensity percentage of Si2p (Fig. 5c) and simultaneous decrease in the percentage C1s (Table 1) indicates that the silica layer formation effectively protects the materials from UV radiation.14
1) matrix before (Fig. 5a) and after (Fig. 5b) UV exposure. After UV irradiation, the structural changes and silica layer formation on the surface of the PDMS–DGEBA polymer matrix can be confirmed by the intensity of C1s, O1s and Si2p atomic percentages. The increase in the intensity percentage of Si2p (Fig. 5c) and simultaneous decrease in the percentage C1s (Table 1) indicates that the silica layer formation effectively protects the materials from UV radiation.14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) before and after UV exposure
1) before and after UV exposure
| Elements | Before UV irradiation | After UV irradiation |
|---|---|---|
| C1s | 50.41 | 47.68 |
| O1s | 18.3 | 19.48 |
| Si2p | 31.29 | 32.67 |
Thermal properties
Thermal degradation temperature and char yield of the PDMS–DGEBA polymer composites were studied by thermogravimetry analysis (TGA) and are presented in Fig. 6. From the TGA thermogram, it can clearly be explained that the PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) polymer composites possess thermal stability up to 270 °C; beyond this temperature, the degradation of the polymer network was observed. This might be due to the cleavage of cyanurate ring and decomposition of organic functionalities. Finally, the char was obtained (Table 2) at 750 °C. Consequently, the PDMS–DGEBA polymer composites can be used as a thermally stable insulating material.
1) polymer composites possess thermal stability up to 270 °C; beyond this temperature, the degradation of the polymer network was observed. This might be due to the cleavage of cyanurate ring and decomposition of organic functionalities. Finally, the char was obtained (Table 2) at 750 °C. Consequently, the PDMS–DGEBA polymer composites can be used as a thermally stable insulating material.
| S. no | Sample | T10 (°C) | Char yield (%) @ 750 °C | Dielectric constant @ 1 MHz (±0.01) | Dielectric loss @ 1 MHz (±0.0001) |
|---|---|---|---|---|---|
| 1 | PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
313 | 13 | 2.87 | 0.0176 |
| 2 | PDMS–DGEBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
306 | 14 | 2.75 | 0.0715 |
UV-shielding properties
Radiation resistant materials are widely used in the field of electronics such as optical electronics and photolithography in aerospace.12,15,25 The PDMS–DGEBA polymer can be used as a good UV shielding material in optical electronics, due to their good transparency, flexibility and UV absorbance/reflectance properties. Fig. 7 shows the UV absorption spectrum of PDMS/DGEBA polymer before and after UV exposed. It indicates that the UV resistant behavior of the PDMS/DGEBA polymer increased by absorbing or reflecting UV rays, whereas the UV treated PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) polymers show (Fig. 7) higher absorbance than those untreated with UV. The shielding efficiency of PDMS–DGEBA to UV rays measured using a UV spectrophotometer is above 200 nm, which is high.26 According to the existing optical theory, the silica particles should possess high scattering capacity, and UV light with wavelength ranging from 200 nm to 400 nm should be scattered by the nano-sized silica.26–28 The transparency of PDMS–DGEBA polymer composite decreased after UV exposure, as shown in Fig. 8a.
1) polymers show (Fig. 7) higher absorbance than those untreated with UV. The shielding efficiency of PDMS–DGEBA to UV rays measured using a UV spectrophotometer is above 200 nm, which is high.26 According to the existing optical theory, the silica particles should possess high scattering capacity, and UV light with wavelength ranging from 200 nm to 400 nm should be scattered by the nano-sized silica.26–28 The transparency of PDMS–DGEBA polymer composite decreased after UV exposure, as shown in Fig. 8a.
Hydrophobic behavior
Contact angle measurements (CA) were carried out using deionized water and diiodomethane (DIM) as the probe liquids. A drop of probe liquid was placed on the surface of the films, and their contact angles were measured and their images were captured (Fig. 9). Fig. 9a and b show the water and DIM contact angles of PDMS–DGEBA. PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PDMS–DGEBA (2
1) and PDMS–DGEBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) show water and DIM contact angles of 83.27° and 32.89° and 92.16° and 35.67°, respectively (Table 3). The surface free energies (SFE) of PDMS–DGEBA polymer composites were calculated (Neumann's method)29,30 from the measured values of contact angles of water and DIM (Table 3). The decreasing value of SFE was observed from PDMS/DGEBA (1
1) show water and DIM contact angles of 83.27° and 32.89° and 92.16° and 35.67°, respectively (Table 3). The surface free energies (SFE) of PDMS–DGEBA polymer composites were calculated (Neumann's method)29,30 from the measured values of contact angles of water and DIM (Table 3). The decreasing value of SFE was observed from PDMS/DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to PDMS/DGEBA (2
1) to PDMS/DGEBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
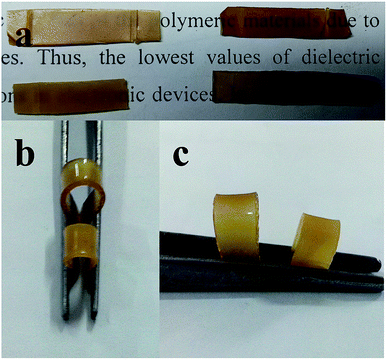
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), indicating that the decreasing value of SFE mainly depends on the PDMS in the PDMS/DGEBA polymer composites. Fig. 8b and c show the images of flexibility of PDMS/DGEBA polymer. Herein, the PDMS/DGEBA (2
1), indicating that the decreasing value of SFE mainly depends on the PDMS in the PDMS/DGEBA polymer composites. Fig. 8b and c show the images of flexibility of PDMS/DGEBA polymer. Herein, the PDMS/DGEBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibits an excellent flexibility, due to the presence of PDMS chain in the polymeric back bone.
1) exhibits an excellent flexibility, due to the presence of PDMS chain in the polymeric back bone.
| Sample | Contact angle (Θ) | Surface free energy (mJ m−2) | |
|---|---|---|---|
| Water | Diiodomethane | ||
PDMS–DGEBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
83.27 | 32.89 | 38.15 |
PDMS–DGEBA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
92.16 | 35.67 | 34.93 |
Dielectric properties
PDMS based polymer composites have been widely used as interlayer low k dielectrics for the fabrication of integrated circuitry devices in microelectronic industries, due to their excellent flexibility, transparency, low dielectric constant and low dielectric loss properties. Moreover, the less polar group of –Si–CH3 present in the PDMS chain significantly contributes to reduction in the value of the dielectric constant by enhancing the hydrophobic behavior of the resulting material.30,31 The earlier reports also support the low dielectric constant behavior of PDMS.30–34 The various types of PDMS, PDMS-PI and PDMS-DDM-PBz polymers show a low dielectric constant value of 3.0,33 3.02 (ref. 34) and 2.96,31 respectively. Thus, the PDMS–DGEBA polymer composites have been developed as low k dielectrics. Fig. 10 shows the decreasing values of dielectric constant as the frequencies increases, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PDMS–DGEBA shows the lower values of dielectric constant (k = 2.75) compared to than that of 1
1 ratio of PDMS–DGEBA shows the lower values of dielectric constant (k = 2.75) compared to than that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PDMS–DGEBA (k = 2.87). From this decreasing value of dielectric constant, it could be clearly inferred that the concentration of PDMS plays a vital role in the reduction in the value of dielectric constant of polymer materials.27 Moreover, the decreasing value of dielectric constant mainly depends on the polarization of the matrix. Hence, the less polar behavior of dimethylsiloxane and triazine units significantly contributes to the reduction in the value of dielectric constant throughout the system. The decreased value of dielectric loss is also one of the important factors to reduce the power consumption and cross talk. Fig. 10b shows the dielectric loss of PDMS–DGEBA composites. The value of dielectric loss decreased while increasing the concentration of PDMS, and 2
1 ratio of PDMS–DGEBA (k = 2.87). From this decreasing value of dielectric constant, it could be clearly inferred that the concentration of PDMS plays a vital role in the reduction in the value of dielectric constant of polymer materials.27 Moreover, the decreasing value of dielectric constant mainly depends on the polarization of the matrix. Hence, the less polar behavior of dimethylsiloxane and triazine units significantly contributes to the reduction in the value of dielectric constant throughout the system. The decreased value of dielectric loss is also one of the important factors to reduce the power consumption and cross talk. Fig. 10b shows the dielectric loss of PDMS–DGEBA composites. The value of dielectric loss decreased while increasing the concentration of PDMS, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PDMS–DGEBA shows the lower value of dielectric loss (0.017).
1 ratio of PDMS–DGEBA shows the lower value of dielectric loss (0.017).
Conclusion
The low k dielectric material of PDMS–DGEBA polymer composite has been developed along with increasing UV resistance, hydrophobicity and thermal stability behaviors. The UV exposed PDMS–DGEBA shows higher shielding property than that of untreated PDMS–DGEBA, due to the formation of a silica layer when treated with UV light for a week, as the silica passive layer effectively protects the materials from UV rays. The dielectric constant and dielectric loss values of PDMS–DGEBA composites were decreased while increasing the concentration of PDMS: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PDMS–DGEBA shows the lower value of dielectric constant of 2.75 at 1 MHz. In addition, the thermal stability increased with an increase in the PDMS content. Hence, the PDMS based polycyanurate composite materials developed in the present study can be used as an insulating material for electronics applications.
1 ratio of PDMS–DGEBA shows the lower value of dielectric constant of 2.75 at 1 MHz. In addition, the thermal stability increased with an increase in the PDMS content. Hence, the PDMS based polycyanurate composite materials developed in the present study can be used as an insulating material for electronics applications.
References
- J. J. Cash, M. C. Davis, M. D. Ford, T. J. Groshens, A. J. Guenthner, B. G. Harvey, K. R. Lamison, J. M. Mabry, H. A. Meylemans, J. T. Reams and C. M. Sahagun, Polym. Chem., 2013, 4, 3859–3865 RSC
.
- J. T. Reams, A. J. Guenthner, K. R. Lamison, G. R. Yandek, D. D. Swanson and J. M. Mabry, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 1061–1070 CrossRef CAS PubMed
.
- I. Hamerton, B. J. Howlin, L. Mooring, C. Stone, M. Swan and S. Thompson, Polym. Degrad. Stab., 2014, 110, 435–446 CrossRef CAS PubMed
.
- A. J. Guenthner, M. E. Wright, A. P. Chafin, J. T. Reams, K. R. Lamison, M. D. Ford, S. P. J. Kirby, J. J. Zavala and J. M. Mabry, Macromolecules, 2014, 47, 7691–7700 CrossRef CAS
.
- M. Ariraman, R. Sasi kumar and M. Alagar, RSC Adv., 2014, 4, 57759–57767 RSC
.
- B. G. Harvey, C. M. Sahagun, A. J. Guenthner, T. J. Groshens, L. R. Cambrea, J. T. Reams and J. M. Mabry, ChemSusChem., 2014, 7, 1964–1969 CrossRef CAS PubMed
.
- B. G. Harvey, A. J. Guenthner, H. A. Meylemans, S. R. L. Haines, K. R. Lamison, T. J. Groshens, L. R. Cambrea, M. C. Davis and W. W. Lai, Green Chem., 2015, 17, 1249–1258 RSC
.
- A. C Hagenaars, C. Bailly, A. Schneider and B. A. Wolf, Polymer, 2002, 43, 2663–2669 CrossRef
.
- P. Thirukumaran, A. Shakila and M. Sarojadevi, RSC Adv., 2014, 4, 7959–7966 RSC
.
- Z. Zhang, G. Liang and X. Wang, Polym. Int., 2014, 63, 552–559 CrossRef CAS PubMed
.
- R. Biju, C. Gouri and C. P. Reghunadhan Nair, Eur. Polym. J., 2012, 48, 499–511 CrossRef CAS PubMed
.
- Y. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550–575 CrossRef CAS
.
- W. Qin, D. Peng, X. Wu and J. Liao, Nucl. Instrum. Methods Phys. Res., Sect. B, 2014, 325, 115–119 CrossRef CAS PubMed
.
- M. Selvi, S. Devaraju, M. R. Vengatesan, J. S. Go, M. Kumar and M. Alagar, RSC Adv., 2014, 4, 8238–8244 RSC
.
- P. Kim, R. Kwak, S. H. Lee and K. Y. Suh, Adv. Mater., 2010, 22, 2426–2429 CrossRef CAS PubMed
.
- Z. Xu, S. Jie and B. G. Li, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 3205–3212 CrossRef CAS PubMed
.
- I. Byun and Be. Kim, Microelectron. Eng., 2014, 121, 92–95 CrossRef CAS PubMed
.
- R. Sasi kumar, M. Ariraman and M. Alagar, RSC Adv., 2015, 5, 23787–23797 RSC
.
- E. M. Maya, A. W. Snow and L. J. Buckley, Macromolecules, 2002, 35, 460–466 CrossRef CAS
.
- R. Sasi kumar, M. Ariraman and M. Alagar, RSC Adv., 2014, 4, 19127–19136 RSC
.
- L. Hu, M. Li, C. Xu, Y. Luo and Y. Zhou, Surf. Coat. Technol., 2009, 203, 3338–3343 CrossRef CAS PubMed
.
- L. Prager, A. Dierdorf, H. Liebe, S. Naumov, S. Stojanović, R. Heller, L. Wennrich and M. R. Buchmeiser, Chem.–Eur. J., 2007, 13, 8522–8529 CrossRef CAS PubMed
.
- R. J. Zaldivar, J. Salfity, G. Steckel, B. Morgan, D. Patel, J. P. Nokes and H. I. Kim, J. Compos. Mater., 2011, 46, 1925–1936 CrossRef PubMed
.
- M. J. Owen and P. J. Smith, J. Adhes. Sci. Technol., 1994, 8, 1063–1075 CrossRef CAS PubMed
.
- J. Lia, Z. Wu, C. Huang and L. Li, Fusion Eng. Des., 2014, 89, 3112–3116 CrossRef PubMed
.
- H. Ma, T. Shi and Q. Song, Fibers, 2014, 2, 275–284 CrossRef CAS PubMed
.
- Y. Zhang, S. Li, F. Huang, F. Wang, W. Duan, J. Li, Y. Shen and A. Xie, Russ. J. Phys. Chem. A, 2012, 86, 413–417 CrossRef CAS
.
- T. Cao, K. Xu, G. Chen and C. Guo, RSC Adv., 2013, 3, 6282–6285 RSC
.
- R. R. Deshmukh and A. R. Shetty, J. Appl. Polym. Sci., 2008, 107, 3707–3717 CrossRef CAS PubMed
.
- D. Shamiryan, T. Abell, F. Iacopi and K. Maex, Mater. Today, 2004, 7, 34–39 CrossRef CAS
.
- R. Sasi kumar, N. Padmanathan and M. Alagar, New J. Chem., 2015, 39, 3995–4008 RSC
.
- X. Zhan, H. Liu, J. Zhang, J. Cheng and X. Lin, Ind. Eng. Chem. Res., 2014, 53, 4254–4262 CrossRef CAS
.
- S. C. B. Mannsfeld, B. C.-K. Tee, R. M. Stoltenberg, C. V. H.-H. Chen, S. Barman, B. V. O. Muir, A. N. Sokolov, C. Reese and Z. Bao, Nat. Mater., 2010, 9, 859–864 CrossRef CAS PubMed
.
- K. Xi, Z. Meng, L. Heng, R. Ge, H. He, X. Yu and X. Jia, J. Appl. Polym. Sci., 2009, 113, 1633–1641 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |