A novel EVA composite with simultaneous flame retardation and ceramifiable capacity
Abstract
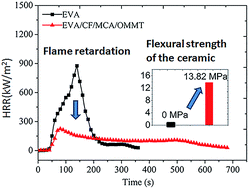
Ethylene-vinyl acetate (EVA) filled with glass dust (GD), glass fiber (GF), OMMT, and melamine cyanurate (MCA) was developed as a ceramifiable flame-retardant polymer composite for cables and insulated wires. The ceramics formed at different high temperatures based on the ceramifiable flame-retardant polymer composites were investigated by mechanical testing, scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) spectroscopy; the flame retardation of the ceramifiable flame-retardant EVA composites was studied with the aid of vertical burning testing (UL-94), limiting oxygen index (LOI), and cone calorimeter (CC) tests. The results showed that the ceramics were prepared successfully at different high temperatures based on the ceramifiable flame-retardant polymer composites. For the EVA/GD/GF/OMMT/MCA system with a weight ratio of 35/26/9/5/25, the ceramic formed at 800 °C had a flexural strength of 13.82 MPa, and both a UL-94 V-0 rating and a LOI of 28.2% were achieved. Moreover, CC results confirmed that the heat release rate (HRR), total release rate (THR), smoke production rate (SPR), and mass loss rate (MLR) of the composite were reduced significantly compared with the corresponding value of neat EVA or the ceramifiable EVA composite without MCA. The dilatometric experiment analysis, SEM, and viscosity analysis demonstrated that a eutectic mixture resulting from GD and GF led to the formation of ceramics at high temperature. Thermal gravimetric analysis (TG), etc. illustrated that both the release of a large amount of non-flammable gas and the presence of OMMT led to the much better flame retardancy of EVA/GD/GF/OMMT/MCA than that of neat EVA or the ceramifiable EVA composites without MCA.

 Please wait while we load your content...
Please wait while we load your content...