DOI:
10.1039/C5RA05780A
(Paper)
RSC Adv., 2015,
5, 52852-52865
Synthesis of biologically important, fluorescence active 5-hydroxy benzo[g]indoles through four-component domino condensations and their fluorescence “Turn-off” sensing of Fe(III) ions†
Received
1st April 2015
, Accepted 8th June 2015
First published on 8th June 2015
Introduction
Nitrogen based heterocycles are important structural motifs for many natural products1 and pharmaceuticals.2 They are also useful building blocks for various biologically active molecules and functional materials.3 As an example, carbazoles, the fused indole based heterocycles, show important applications in materials science as organic light-emitting materials due to their wide band gap and high luminescence activity.4 Fused indole based polycyclic frameworks with proper functionality can provide suitable ligand systems for diverse receptors.5 This type of ligands with fluorescence activity can act as sensing agent for different metal ion through proper binding.6 Form biological point of view sensing of metal ions shows significant demand in current research field.7 More specifically detection of iron level have significant impact in biological research as iron regulates different biological processes such as electron transfer reactions, binding and transport of oxygen, and cell growth and differentiation.8 To the best of our knowledge suitable benzo[g]indole based molecules with fluorescence sensing property have not been discovered as yet. So the development of new synthetic methodology for construction of benzo[g]indole scaffolds is important in organic synthesis. Although there are number of methodologies for the synthesis of fused indole derivatives in literature,9 synthesis of substituted benzo[g]indoles are limited in number.10 Therefore, a simple, efficient, metal-free, regiocontrolled, and diversified synthesis of substituted benzo[g]indole derivatives is still highly desirable. In continuation of our research interest in synthesis of various pyrrole and indole based heterocycles,11 we wish to report herein an efficient synthetic strategy to access fluorescence active 5-hydroxy benzo[g]indoles from easily available starting materials. Since these compounds possess phenolic –OH group as potential binding site the effect of various metal ions on the fluorescence property has also been explored in solution phase. The selective ion sensitive fluorescence changes lead to the finding of fluorescence “Turn-off” sensor of Fe3+ ions.
Results and discussion
During our laboratory efforts on the development of multicomponent reactions for synthesis of biologically important heterocyclic compounds, we discovered that refluxing a mixture of acetylacetone 1 (R1 = Me), aniline 2 (R2 = Ph), phenyl glyoxal (3, R3 = H) and malononitrile (4) in ethanol for 10 minutes produces a new compound 2-(4-acetyl-5-methyl-1,2-diphenyl-1H-3-pyrrolyl)-2-cyanoacetamide 5a in good yield, ∼79% (Scheme 1). The reaction proceeds through a four-component domino condensation among the reactants. Interestingly, under the reaction conditions, one of the two cyano groups of malononitrile is selectively hydrolyzed to an amide group. Subsequently we examine the viability of this reaction in other solvents also such as acetonitrile, dioxane, DMF, THF and DCM for the formation of cyanoacetamide 5a. However ethanol is found to be the best solvent in producing 5a in highest yield (∼79%) whereas acetonitrile produces 5a only in moderate yield (∼40%). The other solvents including dioxane, DMF, THF and DCM produce very negligible amount of 5a. By using the above methodology, a series of 2-pyrrolyl-2-cyanoacetamides 5a–z were synthesized in ethanol medium by varying the 1,3-dicarbonyl compounds, amines as well as the arylglyoxal monohydrates (Scheme 1, Table 1). The results show that this four-component domino condensation reaction provides an elegant and rapid way to access various 2-pyrrolyl-2-cyanoacetamides in a single synthetic operation from simple building blocks.
 |
| | Scheme 1 Synthesis of 2-pyrrolyl-2-cyanoacetamides 5 through domino condensation. | |
Table 1 Synthesis of 2-pyrrolyl-2-cyanoacetamides 5 in ethanol
| Entry |
R1 |
R2 |
R3 |
Product |
Yielda (%) |
M.p. |
| Isolated yield. Compounds 5y and 5z were not possible to isolate because of closely spaced impurities. The crude masses were used for further reaction. |
| 1 |
Me |
 |
H |
5a |
79 |
160–162 |
| 2 |
Me |
 |
H |
5b |
76 |
168–170 |
| 3 |
Me |
 |
H |
5c |
73 |
146–148 |
| 4 |
Me |
 |
H |
5d |
74 |
180–182 |
| 5 |
Me |
 |
H |
5e |
71 |
218–220 |
| 6 |
Me |
 |
H |
5f |
82 |
162–164 |
| 7 |
Me |
 |
H |
5g |
77 |
196–198 |
| 8 |
Me |
 |
H |
5h |
81 |
166–168 |
| 9 |
Me |
 |
Cl |
5i |
73 |
118–120 |
| 10 |
Me |
 |
Cl |
5j |
72 |
198–200 |
| 11 |
Me |
 |
Cl |
5k |
73 |
150–152 |
| 12 |
Me |
 |
Cl |
5l |
79 |
184–186 |
| 13 |
Me |
 |
Cl |
5m |
74 |
240–242 |
| 14 |
Me |
 |
Cl |
5n |
71 |
244–246 |
| 15 |
Me |
 |
Cl |
5o |
72 |
205–207 |
| 16 |
Me |
 |
Cl |
5p |
76 |
208–210 |
| 17 |
Me |
 |
OMe |
5q |
81 |
164–166 |
| 18 |
Me |
 |
OMe |
5r |
75 |
198–200 |
| 19 |
Me |
 |
OMe |
5s |
78 |
88–90 |
| 20 |
Me |
 |
OMe |
5t |
81 |
152–154 |
| 21 |
CO2Et |
 |
H |
5u |
78 |
164–166 |
| 22 |
CO2Et |
 |
H |
5v |
75 |
194–196 |
| 23 |
CO2Et |
 |
H |
5w |
79 |
216–218 |
| 24 |
CO2Et |
 |
H |
5x |
73 |
78–80 |
| 25 |
Ph |
 |
H |
5y |
Impureb |
— |
| 26 |
Ph |
 |
H |
5z |
Impureb |
— |
A proposed mechanism for the formation of compound 5 is shown in Scheme 2.12 Initially, a Knoevenagel condensation between arylglyoxal monohydrate 3 and malononitrile produces α,β-unsaturated cyano intermediate 6. Then enamine 7, derived in situ from acetylacetone 1 and amines 2, undergoes a Michael-type 1,4-addition with the intermediate 6 to produce imminium intermediate 8, which subsequently tautomerizes to 9. Then the intermediate 9 most probably produces a fused furano-pyrrole intermediate 10 through a tandem cyclization process. Finally, the 2-pyrrolyl-2-cyanoacetamides 5 are formed through a rearrangement of intermediate 10. All the compounds 5a–x were fully characterized by 1H and 13C NMR spectroscopy, and the X-ray crystal structure analysis of 2-[4-acetyl-2-(4-chlorophenyl)-5-methyl-p-tolyl-1H-3-pyrrolyl]-2-cyanoacetamide (5j) further confirmed the structural assignment (Fig. 1).13
 |
| | Scheme 2 Plausible mechanism for the formation of 5. | |
 |
| | Fig. 1 ORTEP diagram of the X-ray crystal structure of compound 5j with the atom numbering scheme; thermal ellipsoids are shown at the 50% probability. | |
After successful synthesis of 2-pyrrolyl-2-cyanoacetamides 5 we focussed our attention to explore the chemical property of this substance. Therefore, as a preliminary study, 2-(4′-acetyl-5-methyl-1,2-diphenyl-1H-3-pyrrolyl)-2-cyanoacetamide 5a was heated at reflux in diphenyl ether to examine the formation of any cyclic product. Interestingly, a cyclic product 3-acetyl-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile 11a was formed in moderate yield (∼61%) within 10 minutes through a thermal cyclization (Scheme 3). By employing this thermal method a series of biologically important 5-hydroxy benzo[g]indoles 11 were synthesized (Table 2). This novel thermal cyclization also provides an excellent opportunity to synthesise differently substituted 5-hydroxy benzo[g]indoles. Therefore we synthesized a series of cyanoacetamide derivatives 12 following a different reaction protocol12 and converted them to the corresponding 5-hydroxy benzo[g]indoles 13 by refluxing in diphenyl ether for 10 minutes (Scheme 4, Table 3). All the compounds 11 and 13 were fully characterized by 1H and 13C NMR data. Further determination of X-ray crystal structure of 3-acetyl-5-hydroxy-1-cyclopropyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11h) and 3-carboethoxy-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11u) confirmed the product formation (Fig. 2).13
 |
| | Scheme 3 Synthesis of benzo[g]indole derivatives 11 through thermal cyclization. | |
Table 2 Synthesis of 5-hydroxy benzo[g]indole derivatives 11
| Entry |
R1 |
R2 |
R3 |
Starting |
Product |
Yielda (%) |
M. p. |
| Isolated yield. For compound 11y and 11z, the yields are calculated with respect to starting material 1. |
| 1 |
Me |
 |
H |
5a |
11a |
61 |
228–230 |
| 2 |
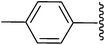
Me |
 |
H |
5b |
11b |
55 |
238–240 |
| 3 |
Me |
 |
H |
5c |
11c |
52 |
212–214 |
| 4 |
Me |
 |
H |
5d |
11d |
53 |
253–255 |
| 5 |
Me |
 |
H |
5e |
11e |
51 |
247–249 |
| 6 |
Me |
 |
H |
5f |
11f |
59 |
140–142 |
| 7 |
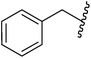
Me |
 |
H |
5g |
11g |
60 |
202–204 |
| 8 |
Me |
 |
H |
5h |
11h |
62 |
194–196 |
| 9 |
Me |
 |
Cl |
5i |
11i |
53 |
270–272 |
| 10 |
Me |
 |
Cl |
5j |
11j |
51 |
238–240 |
| 11 |
Me |
 |
Cl |
5k |
11k |
56 |
178–180 |
| 12 |
Me |
 |
Cl |
5l |
11l |
54 |
208–210 |
| 13 |
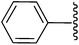
Me |
 |
OMe |
5q |
11q |
52 |
188–190 |
| 14 |
CO2Et |
 |
H |
5u |
11u |
59 |
218–220 |
| 15 |
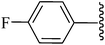
CO2Et |
 |
H |
5v |
11v |
56 |
246–248 |
| 16 |
CO2Et |
 |
H |
5w |
11w |
61 |
256–258 |
| 17 |
CO2Et |
 |
H |
5x |
11x |
55 |
192–194 |
| 18 |
Ph |
 |
H |
5y |
11yb |
41 |
256–258 |
| 19 |
Ph |
 |
H |
5z |
11zb |
43 |
248–250 |
 |
| | Scheme 4 Synthesis of 2,3-dicarboethoxy-5-hydroxy-1-aryl-1H-benzo[g]indole-4-carbonitrile 13 through thermal cyclization. | |
Table 3 Synthesis of 2,3-dicarboethoxy-5-hydroxy-1-aryl-1H-benzo[g]indole-4-carbonitrile 13
| Entry |
R |
Starting |
Product |
Yielda (%) |
M. p. |
| Isolated yield. |
| 1 |
 |
12a |
13a |
64 |
180–182 |
| 2 |
 |
12b |
13b |
52 |
168–170 |
| 3 |
 |
12c |
13c |
61 |
238–240 |
| 4 |
 |
12d |
13d |
60 |
194–196 |
| 5 |
 |
12e |
13e |
63 |
180–182 |
| 6 |
 |
12f |
13f |
55 |
176–178 |
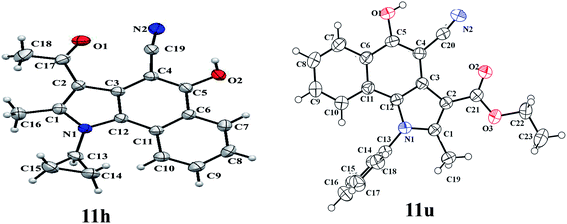 |
| | Fig. 2 ORTEP diagrams of the X-ray crystal structures of compounds 11h and 11u with the atom numbering scheme; thermal ellipsoids are shown at the 50% probability. | |
After successful synthesis of various substituted 5-hydroxy benzo[g]indoles 11/13 we focussed our attention in exploring the photochemical property of this substance. Steady state absorption and emission spectra of some of the selective compounds 11u–w have been taken in different solvents with varying polarity such as CH2Cl2, CH3CN and CH3OH. The concentration of the compounds 11 was maintained at ∼5 × 10−5 M. UV-Vis absorption spectrum shows structured band with peaks around 369 nm, 356 nm (Fig. 3a, Table 4). The emission spectrum shows a structure less band with maxima around 408 nm (Fig. 3b, Table 4). The shape and band position of the emission spectra are same regardless of excitation wavelength. Noticeably the molecules display significant Stokes shift (Table 4) which indicates that the structure of the emitting species and the ground state species are considerably different. The measured fluorescence quantum yields (ΦF) of some of the representative compounds are found around ∼0.5 which does not change much with the polarity of the solvent (Table 4).
 |
| | Fig. 3 (a) UV-Vis absorption spectra and (b) fluorescence emission spectra (λ in nm) of representative compound 11u in different solvents ([11u] at ∼5 × 10−5 M conc.). | |
Table 4 Spectroscopic data of 11 in three solvents
| Compounds |
Solvents |
λabs (nm) |
λem (nm) |
εmaxa (104 cm−1 mol−1) |
ΦFb |
Stokes shiftc (104 cm−1) |
ΔEd (eV) |
| εmax is the extinction coefficient at λmax of absorption. Fluorescence quantum yields with reference to β-naphthol in methyl cyclohexane (ΦF 0.53). Stokes shift = (1/λabs − 1/λem). Determined from UV-Vis absorption maximum. |
| 11u |
CH2Cl2 |
370 |
407 |
0.82 |
0.57 |
0.24 |
3.35 |
| CH3CN |
369 |
406 |
0.65 |
0.50 |
0.25 |
3.36 |
| CH3OH |
368 |
410 |
0.58 |
0.49 |
0.27 |
3.37 |
| 11v |
CH2Cl2 |
371 |
404 |
0.94 |
0.51 |
0.22 |
3.34 |
| CH3CN |
368 |
408 |
0.68 |
0.49 |
0.26 |
3.37 |
| CH3OH |
369 |
407 |
0.59 |
0.48 |
0.25 |
3.36 |
| 11w |
CH2Cl2 |
373 |
410 |
0.85 |
0.54 |
0.24 |
3.32 |
| CH3CN |
369 |
407 |
0.71 |
0.51 |
0.25 |
3.36 |
| CH3OH |
370 |
406 |
0.59 |
0.50 |
0.24 |
3.35 |
The photo-physical properties of the compound 11u as a ligand have been explored in detail in presence of various metal ions Na+, Ba2+, Hg2+, Cd2+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+ in acetonitrile. The complexation of ligand 11u with Fe3+ ions in acetonitrile was accompanied by a change of colour of the solution from colourless to yellow which was easily observed by the naked-eye (Fig. 4A). On addition of Cu2+ ions a very light green colour was developed. The developing colour was more intense in case Fe3+ than in Cu2+ under similar conditions. The addition of 5 equiv. of other cations as their perchlorate salts resulted in no appreciable changes in colour under visible light (Fig. 4A). Interestingly, under UV light substantial fluorescence quenching is observed only in presence of Fe3+ ions (Fig. 4B). UV-Vis spectra of the ligand 11u in acetonitrile show no significant change on addition of metal perchlorate salts of Na+, Ba2+, Hg2+, Cd2+, Mn2+, Co2+, Ni2+, Zn2+ (ESI, Fig. S5†). However in presence of Fe3+ and Cu2+ the UV-Vis spectrum of 11u shows appreciable change compared to that of free ligand hence indicating ligand–metal interaction. On gradual addition of Fe3+ salt an absorption band develops near 400 nm with simultaneous appearance of two isobestic points at 303 nm and 380 nm (Fig. 5). The emission spectra of ligand 11u in acetonitrile shows no change in fluorescence intensity in presence of metal ions Na+, Ba2+, Hg2+, Cd2+, Mn2+, Co2+, Ni2+, Zn2+(Fig. 6). On the other hand substantial quenching of ligand emission is observed in presence of Fe3+ and less extent of quenching is observed in presence of Cu2+ (Fig. 6). The result indicates that the extent of fluorescence quenching in Fe3+ is significantly higher compared to that of Cu2+ (Fig. 6). Further in fluorometric titration nearly complete fluorescence quenching is observed on addition of ∼1.2 equivalent of Fe3+ ions (Fig. 7). The high selectivity of 11u for Fe3+ may be due to the presence of more charges (3+) on iron ions compared to that of other metal ions (2+ or 1+), which probably causes strongest interaction with the phenolate anion. The detection limit of Fe3+ by probe 11u has been estimated from fluorescence titration and found to be ∼1.2 × 10−6 M (ESI, Fig. S6†). From the same titration, the binding constant of probe 11u with Fe3+ metal ion has been measured as ∼7.97 × 103 M−1 using Benesi–Hildebrand equation (see ESI, Fig. S7†). The metal (Fe3+) to ligand (11u) binding ratio has also been calculated by jobs plot method using UV titration and found to be in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see ESI, Fig. S8†).
1 ratio (see ESI, Fig. S8†).
 |
| | Fig. 4 Change in color of solution of 11u in acetonitrile in presence of 5 equiv. of each metal ion (A) under visible light and (B) under UV light. | |
 |
| | Fig. 5 Absorption spectra of 11u ([11u] = ∼5 × 10−5 M) on increasing concentration of Fe3+ ions in acetonitrile. | |
 |
| | Fig. 6 Emission spectra of 11u in acetonitrile in presence of various metal ions ([11u] at 5 × 10−5 M conc.). | |
 |
| | Fig. 7 Emission spectra of 11u in acetonitrile on gradual addition of Fe3+ ions ([11u] at 5 × 10−5 M conc.). | |
Subsequently the fluorescence “Turn-off” sensing of probe 11u for Fe3+ ions has been examined in presence of other metal ions. When Fe3+ ions were added to various solutions of 11u containing one of the different metal ions Co2+, Ba2+, Cd2+, Cu2+, Hg2+, Mn2+, Na+, Ni2+, Zn2+ in acetonitrile the binding of Fe3+ ions with 11u was not significantly affected by the presence of these other metal ions (Fig. 8). Therefore, the intensity of the fluorescence band of the complex [11u–Fe3+] at 408 nm can be used to monitor the presence of Fe3+ ions alone as well as Fe3+ ions in presence of other metal ions.
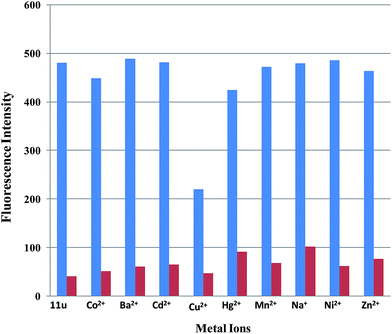 |
| | Fig. 8 The selectivity of 11u (5 × 10−5 M conc.) in the presence of various metal ions in acetonitrile. The blue bars represent the emission intensity of 11u in the presence of other metal ions (25 × 10−5 M conc.). The red bars represent the emission intensity that occurs upon the subsequent addition of Fe3+ ions (5 × 10−5 M conc.) to the above solution (λem = 408 nm). | |
In order to get more insights about the nature of Fe3+ ligand interactions 1H NMR titration was carried out. In this experiment the perchlorate salt of Fe3+ ions was gradually added to a DMSO-d6 solution of 11u. The results show that the intensity of the proton signal corresponding to 5-hydroxyl group (–OH) of 11u at 10.86 ppm, which is the most probable binding site for Fe3+ ions, decreases significantly relative to that of other protons upon exposure to Fe3+ (Fig. 9). This effect proves unambiguously the formation of complex between 11u and Fe3+ ion. Additionally, a rapid broadening and finally disappearance of the proton signal at 10.86 ppm was observed with gradual addition of Fe3+ ions which further confirms the fast exchange of hydroxyl (–OH) protons due to complexation with Fe3+ ion. Moreover during the NMR titration a light yellow colour was developed initially which gradually intensified with addition of more and more perchlorate salt of Fe3+ ions to the DMSO-d6 solution of 11u indicating complexation between Fe3+ ion and 11u. The UV-Vis and NMR data clearly suggest that the phenolic hydroxyl groups (–OH) of 11u are deprotonated due to complexation with Fe3+ ions which is probably responsible for strong fluorescence quenching.
 |
| | Fig. 9 Change in 1H NMR spectra of 11u upon addition of Fe3+ ions, (a) 00 equiv., (b) 0.5 equiv., (c) 1.0 equiv., and (d) 2.0 equiv. in [d6]-DMSO. | |
Conclusions
In conclusion, a convenient two steps methodology has been developed for the synthesis of biologically important 5-hydroxy benzo[g]indoles from various easily available 1,3-dicarbonyl compounds, amines, arylglyoxals and malononitrile under metal free conditions. The present synthetic protocol is quite atom economical in nature since only two molecules of water and one molecule of ammonia are released during the course of the reactions. The synthesised 5-hydroxy benzo[g]indoles show fluorescence activity with good quantum yields (ΦF = ∼0.50) and ability to act as fluorescence “Turn-off” sensor for Fe3+ ions. The interaction of 5-hydroxy benzo[g]indoles with Fe3+ ions can also be monitored through UV-Vis spectral change and naked-eye colour change in the presence and absence of UV radiation. Thus, the sensing of 5-hydroxy benzo[g]indoles for iron at the low level (detection limit = ∼1.2 × 10−6 M) may have potential applications in current biomedical and pathological research works. It is also worth mentioning that phenolics containing iron-binding motifs have also been identified in many bio-active plants such as grapes, tea and traditional Chinese medicine plants.
Experimental section
General
Arylglyoxals 3 were prepared from corresponding acetophenones by oxidation with selenium dioxide in dioxane.14 Solvents were purchased from commercial suppliers and used after distillation. Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded with a Perkin-Elmer 782 spectrophotometer. 1H (300 MHz) and 13C (75 MHz) NMR spectra were recorded in DMSO-d6 with a Bruker 300 MHz instrument. Elemental analyses (C, H and N) were performed by using a Perkin-Elmer 240C elemental analyzer. The X-ray diffraction study of crystallized compounds was performed with Bruker APEX-II CCD system.
2-pyrrolyl-2-cyanoacetamides 5a–z; general procedure
A mixture of 1,3-dicarbonyl compound 1 (1.0 mmol) and amine 2 (1.0 mmol) in ethanol (20 mL) was heated for 2 minutes. Then to the above hot mixture, arylglyoxal 3 (1.0 mmol) and malononitrile 4 (1.3 mmol) were added and the reaction mixture was heated at reflux for 10 minutes. After completion of the reaction (monitored by TLC analysis), the solvent was removed under reduced pressure. The resulting solid residues were purified by column chromatography (EtOAc–hexane) on silica gel to get the light yellow solid products 5.
2-(4-Acetyl-5-methyl-1,2-diphenyl-1H-3-pyrrolyl)-2-cyanoacetamide (5a). Light yellow solid; yield: 282 mg (79%); m.p.: 160–162 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.40–7.30 (m, 4H), 7.28–7.21 (m, 5H), 7.12–7.07 (m, 3H), 4.73 (s, 1H), 2.47 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.6, 137.3, 136.7, 135.3, 131.1, 130.0, 129.5, 129.3, 129.0, 128.6, 128.5, 120.4, 117.9, 111.8, 37.2, 31.1, 14.2 ppm; IR (KBr): 2378, 1705, 1650 cm−1; anal. calcd for C22H19N3O2: C, 73.93; H, 5.36; N, 11.76% found C, 73.85; H, 5.30; N, 11.69%.
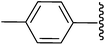
2-(4-Acetyl-5-methyl-2-phenyl-1-p-tolyl-1H-3-pyrrolyl)-2-cyanoacetamide (5b). Light yellow solid; yield: 281 mg (76%); m.p.: 168–170 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.37 (s, 1H), 7.31–7.24 (m, 3H), 7.22–7.12 (m, 6H), 7.06 (s, 1H), 4.73 (s, 1H), 2.47 (s, 3H), 2.32 (s, 3H), 2.25 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.6, 138.4, 136.8, 135.4, 134.7, 131.1, 130.1, 129.9, 128.9, 128.6, 128.5, 120.3, 117.9, 111.7, 37.2, 31.3, 21.0, 14.3 ppm; IR (KBr): 2376, 1708, 1647 cm−1; anal. calcd for C23H21N3O2: C, 74.37; H, 5.70; N, 11.31% found C, 74.30; H, 5.62; N, 11.23%.
2-[4-Acetyl-5-methyl-1-(4-bromophenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5c). Light yellow solid; yield: 317 mg (73%); m.p.: 146–148 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.58 (d, J = 5.4 Hz, 2H), 7.38 (s, 1H), 7.27–7.17 (m, 5H), 7.13–7.10 (m, 2H), 7.05 (s, 1H), 4.69 (s, 1H), 2.47 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.5, 136.7, 135.2, 132.5, 131.4, 131.1, 129.8, 128.8, 128.7, 122.2, 120.6, 117.8, 112.0, 107.8, 37.1, 31.1, 14.2 ppm; IR (KBr): 2370, 1701, 1649 cm−1; anal. calcd for C22H18BrN3O2: C, 60.56; H, 4.16; N, 9.63% found C, 60.48; H, 4.10; N, 9.55%.
2-[4-Acetyl-5-methyl-1-(4-chlorophenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5d). Light yellow solid; yield: 289 mg (74%); m.p.: 180–182 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.46–7.40 (m, 4H), 7.28–7.21 (m, 4H), 7.16–7.14 (m, 2H), 7.08 (s, 1H), 4.74 (s, 1H), 2.50 (s, 3H), 2.36 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.6, 136.7, 136.3, 135.3, 133.7, 131.2, 129.8, 129.5, 128.7, 120.6, 117.9, 112.0, 37.2, 31.2, 14.2 ppm; IR (KBr): 2372, 1704, 1647 cm−1; anal. calcd for C22H18ClN3O2: C, 67.43; H, 4.63; N, 10.72% found C, 67.34; H, 4.55; N, 10.65%.
2-[4-Acetyl-5-methyl-1-(4-fluorophenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5e). Light yellow solid; yield: 266 mg (71%); m.p.: 218–220 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.40–7.36 (m, 2H), 7.28–7.20 (m, 6H), 7.15–7.13 (m, 2H), 7.07 (s, 1H), 4.73 (s, 1H), 2.49 (s, 3H), 2.35 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.6, 163.5, 160.3, 136.9, 135.5, 133.7, 133.6, 131.5, 131.2, 129.9, 128.8, 128.6, 120.4, 117.9, 116.5, 116.2, 111.8, 37.2, 31.1, 14.2 ppm; IR (KBr): 2371, 1701, 1652 cm−1; anal. calcd for C22H18FN3O2: C, 70.39; H, 4.83; N, 11.19% found C, 70.31; H, 4.76; N, 11.12%.
2-[4-Acetyl-5-methyl-1-(4-methoxyphenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5f). Light yellow solid; yield: 317 mg (82%); m.p.: 162–164 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.33 (s, 1H), 7.27–7.21 (m, 4H), 7.15–7.12 (m, 3H), 7.05 (s, 1H), 6.92 (d, J = 8.7 Hz, 2H), 4.72 (s, 1H), 3.73 (s, 3H), 2.48 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.6, 159.3, 137.0, 135.5, 131.2, 130.3, 130.2, 128.6, 128.5, 120.2, 118.0, 116.5, 114.6, 114.6, 111.6, 55.7, 37.2, 31.1, 14.2 ppm; IR (KBr): 2370, 1706, 1656 cm−1; anal. calcd for C23H21N3O3: C, 71.30; H, 5.46; N, 10.85% found C, 71.22; H, 5.38; N, 10.77%.
2-(4-Acetyl-5-methyl-1-benzyl-2-phenyl-1H-3-pyrrolyl)-2-cyanoacetamide (5g). Light yellow solid; yield: 285 mg (77%); m.p.: 196–198 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.32–7.31 (m, 3H), 7.23–7.11 (m, 6H), 6.88–6.84 (m, 3H), 4.97 (s, 2H), 4.70 (s, 1H), 2.35 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.5, 137.6, 136.1, 135.0, 131.2, 129.8, 129.5, 129.1, 127.7, 126.1, 120.5, 118.1, 111.7, 47.7, 37.0, 31.3, 13.4 ppm; IR (KBr): 2375, 1703, 1646 cm−1; anal. calcd for C23H21N3O2: C, 74.37; H, 5.70; N, 11.31% found C, 74.30; H, 5.64; N, 11.24%.
2-(4-Acetyl-5-methyl-1-cyclopropyl-2-phenyl-1H-3-pyrrolyl)-2-cyanoacetamide (5h). Light yellow solid; yield: 260 mg (81%); m.p.: 166–168 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.49–7.44 (m, 3H), 7.41–7.35 (m, 2H), 7.25 (s, 1H), 6.84 (s, 1H), 4.68 (s, 1H), 3.26–3.20 (m, 1H), 2.67 (s, 3H), 2.42 (s, 3H), 0.77–0.69 (m, 2H), 0.44–0.43 (m, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.5, 166.7, 138.7, 135.6, 130.9, 130.7, 128.8, 128.7, 119.7, 118.0, 110.7, 37.0, 31.2, 27.5, 14.2, 9.6, 9.5 ppm; IR (KBr): 2371, 1701, 1652 cm−1; anal. calcd for C19H19N3O2: C, 71.01; H, 5.96; N, 13.08% found C, 69.92; H, 5.90; N, 13.01%.
2-[4-Acetyl-5-methyl-1-phenyl-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5i). Light yellow solid; yield: 285 mg (73%); m.p.: 118–120 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.39–7.34 (m, 4H), 7.33–7.31 (m, 4H), 7.16–7.13 (m, 3H), 4.90 (s, 1H), 2.49 (s, 3H), 2.35 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.1, 166.5, 137.1, 136.9, 133.9, 133.6, 133.0, 129.6, 129.2, 129.1, 128.6, 120.5, 117.9, 112.2, 36.9, 31.2, 14.2 ppm; IR (KBr): 2372, 1704, 1647 cm−1; anal. calcd for C22H18ClN3O2: C, 67.43; H, 4.63; N, 10.72% found C, 67.35; H, 4.54; N, 10.63%.
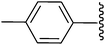
2-[4-Acetyl-5-methyl-1-p-tolyl-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5j). Light yellow solid; yield: 292 mg (72%); m.p.: 198–200 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.39 (s, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.22–7.13 (m, 7H), 4.88 (s, 1H), 2.48 (s, 3H), 2.33 (s, 3H), 2.30 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.5, 138.7, 137.0, 134.5, 133.9, 133.6, 133.0, 130.1, 129.1, 128.9, 128.6, 120.4, 112.1, 107.8, 36.9, 31.2, 21.0, 14.2 ppm; IR (KBr): 2376, 1707, 1649 cm−1; anal. calcd for C23H20ClN3O2: C, 68.06; H, 4.97; N, 10.35% found, 67.98; H, 4.91; N, 10.27%.
2-[4-Acetyl-5-methyl-1-benzyl-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5k). Light yellow solid; yield: 296 mg (73%); m.p.: 150–152 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.47 (d, J = 8.4 Hz, 2H), 7.31–7.24 (m, 6H), 7.07 (s, 1H), 6.93 (d, J = 7.2 Hz, 2H), 5.06 (s, 2H), 4.97 (s, 1H), 2.46 (s, 3H), 2.43 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.2, 166.4, 137.5, 136.3, 134.4, 133.5, 133.1, 129.1, 128.8, 127.7, 126.0, 120.6, 118.0, 112.2, 47.7, 36.6, 31.4, 13.4 ppm; IR (KBr): 2375, 1704, 1653 cm−1; anal. calcd for C23H20ClN3O2: C, 68.06; H, 4.97; N, 10.35% found, 67.95; H, 4.90; N, 10.28%.
2-[4-Acetyl-5-methyl-1-cyclopropyl-2-(4′-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5l). Light yellow solid; yield: 281 mg (79%); m.p.: 184–186 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.31 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.1 Hz, 2H), 7.07 (s 1H), 6.70 (s, 1H), 4.61 (s, 1H), 3.05–3.02 (m, 1H), 2.46 (s, 3H), 2.21 (s, 3H), 0.62–0.58 (m, 2H), 0.25–0.23 (m, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.7, 166.6, 139.0, 134.3, 133.5, 132.6, 129.9, 128.8, 119.8, 117.9, 111.2, 36.7, 31.4, 27.5, 14.5, 14.2, 9.6 ppm; IR (KBr): 2370, 1702, 1657 cm−1; anal. calcd for C19H18ClN3O2: C, 64.13; H, 5.10; N, 11.81% found, C, 64.07; H, 5.04; N, 11.74%.
2-[4-Acetyl-5-methyl-1-(4-bromophenyl)-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5m). Light yellow solid; yield: 347 mg (74%); m.p.: 240–242 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.64–7.61 (m, 2H), 7.41 (s, 1H), 7.37–7.35 (m, 2H), 7.30–7.22 (m, 2H), 7.17–7.11 (m, 3H), 4.87 (s, 1H), 2.49 (s, 3H), 2.35 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.5, 136.9, 133.8, 133.0, 132.6, 131.4, 128.8, 122.4, 120.7, 117.8, 112.4, 36.9, 31.2, 14.2 ppm; IR (KBr): 2371, 1709, 1643 cm−1; anal. calcd for C22H17BrClN3O2: C, 56.13; H, 3.64; N, 8.93% found C, 56.06; H, 3.58; N, 8.85%.
2-[4-Acetyl-5-methyl-1,2-bis-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5n). Light yellow solid; yield: 302 mg (71%); m.p.: 244–246 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.47 (d, J = 8.7 Hz, 2H), 7.41 (s, 1H), 7.35–7.26 (m, 4H), 7.14–7.10 (m, 3H), 4.84 (s, 1H), 2.47 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.5, 137.0, 136.0, 133.9, 133.8, 133.0, 131.2, 131.1, 129.7, 128.8, 120.7, 117.8, 112.4, 36.9, 31.2, 14.2 ppm; IR (KBr): 2367, 1702, 1648 cm−1; anal. calcd for C22H17Cl2N3O2: C, 61.98; H, 4.02; N, 9.86% found C, 61.91; H, 3.95; N, 9.80%.
2-[4-Acetyl-5-methyl-1-(4-fluorophenyl)-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5o). Light yellow solid; yield: 295 mg (72%); m.p.: 205–207 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.41–7.26 (m, 7H), 7.17–7.12 (m, 3H), 4.88 (s, 1H), 2.50 (s, 3H), 2.35 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.5, 137.1, 134.0, 133.7, 133.4, 133.0, 131.5, 128.9, 128.7, 120.5, 117.8, 116.7, 116.4, 112.2, 36.9, 31.2, 14.2 ppm; IR (KBr): 2371, 1709, 1643 cm−1; anal. calcd for C22H17ClFN3O2: C, 64.47; H, 4.18; N, 10.25% found C, 64.41; H, 4.12; N, 10.18%.
2-[4-Acetyl-5-methyl-1-(4-methoxyphenyl)-2-(4-chlorophenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5p). Light yellow solid; yield: 320 mg (76%); m.p.: 208–210 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.39–7.32 (m, 3H), 7.20–7.11 (m, 5H), 6.94 (d, J = 8.1 Hz, 2H), 4.88 (s, 1H), 3.74 (s, 3H), 2.48 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 194.0, 166.6, 159.4, 137.2, 134.1, 133.6, 133.0, 130.4, 130.3, 129.7, 129.2, 128.6, 120.3, 117.9, 114.7, 112.0, 55.7, 36.9, 31.2, 14.2 ppm; IR (KBr): 2375, 1702, 1640 cm−1; anal. calcd for C23H20ClN3O3: C, 65.48; H, 4.78; N, 9.96% found C, 65.41; H, 4.72; N, 9.89%.
2-[4-Acetyl-5-methyl-1-phenyl-2-(4-methoxyphenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5q). Light yellow solid; yield: 313 mg (81%); m.p.: 164–166 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.38–7.30 (m, 4H), 7.28–7.23 (m, 2H), 7.05–7.02 (m, 3H), 6.79 (d, J = 8.7 Hz, 2H), 4.71 (s, 1H), 3.66 (s, 3H), 2.47 (s, 3H), 2.31 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.8, 166.7, 159.4, 137.4, 136.4, 135.2, 132.5, 129.5, 129.0, 122.0, 120.3, 118.0, 114.0, 111.6, 55.4, 37.2, 31.1, 14.2 ppm; IR (KBr): 2369, 1701, 1655 cm−1; anal. calcd for C23H21N3O3: C, 71.30; H, 5.46; N, 10.85% found C, 71.21; H, 5.36; N, 10.75%.
2-[4-Acetyl-5-methyl-1-(4-chlorophenyl)-2-(4-methoxyphenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5r). Light yellow solid; yield: 316 mg (75%); m.p.: 198–200 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.48–7.25 (m, 5H), 7.07–6.98 (m, 3H), 6.92–6.82 (m, 2H), 4.70 (s, 1H), 3.69 (s, 3H), 2.47 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.5, 166.4, 159.3, 136.2, 135.0, 133.4, 132.3, 130.9, 129.3, 128.6, 121.5, 120.2, 117.7, 113.9, 111.6, 55.2, 37.0, 30.9, 13.9 ppm; IR (KBr): 2371, 1704, 1643 cm−1; anal. calcd for C23H20ClN3O3: C, 65.48; H, 4.78; N, 9.96% found C, 65.40; H, 4.71; N, 9.87%.
2-[4-Acetyl-5-methyl-1-benzyl-2-(4-methoxyphenyl)-1H-3-pyrrolyl]-2-cyanoacetamide (5s). Light yellow solid; yield: 312 mg (78%); m.p.: 88–90 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.33–7.28 (m, 4H), 7.25–7.17 (m, 2H), 6.97–6.95 (m, 5H), 5.04 (s, 2H), 4.78 (s, 1H), 3.74 (s, 3H), 2.43 (s, 3H), 2.40 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.7, 166.4, 159.9, 137.5, 135.6, 134.7, 132.4, 128.9, 127.4, 125.9, 121.5, 120.2, 117.9, 114.3, 111.5, 55.3, 36.8, 31.1, 13.2 ppm; IR (KBr): 2374, 1705, 1657 cm−1; anal. calcd for C24H23N3O3: C, 71.80; H, 5.77; N, 10.47% found, C, 71.72; H, 5.71; N, 10.40%.
2-[4-Acetyl-5-methyl-1-cyclopropyl-2-(4-methoxyphenyl)-1H-3-pyrrolyl]-2-cyano acetamide (5t). Light yellow solid; yield: 284 mg (81%); m.p.: 152–154 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.68 (d, J = 8.1 Hz, 3H), 7.01 (d, J = 7.8 Hz, 2H), 6.82 (s, 1H), 4.65 (s, 1H), 3.78 (s, 3H), 3.20–3.10 (m, 1H), 2.65 (s, 3H), 2.40 (s, 3H), 0.92–0.90 (m, 2H), 0.46–0.44 (m, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 193.5, 166.7, 159.5, 138.4, 135.6, 132.1, 123.0, 119.6, 118.1, 114.2, 110.6, 55.5, 37.0, 31.2, 27.4, 14.1, 9.4 ppm; IR (KBr): 2368, 1704, 1652 cm−1; anal. calcd for C20H21N3O3: C, 68.36; H, 6.02; N, 11.96% found, C, 68.28; H, 5.94; N, 11.90%.
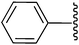
2-(4-Carboethoxy-5-methyl-1,2-diphenyl-1H-3-pyrrolyl)-2-cyanoacetamide (5u). Light yellow solid; yield: 302 mg (78%); m.p.: 164–166 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.45 (s, 1H), 7.30–7.27 (bs, 4H), 7.18–7.17 (m, 4H), 7.09–7.05 (m, 3H), 4.57 (s, 1H), 4.18–4.11 (m, 2H), 2.41 (s, 3H), 1.25–1.20 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 161.0, 158.7, 132.3, 131.5, 129.3, 125.3, 124.2, 123.7, 123.2, 122.8, 122.5, 112.4, 105.8, 104.3, 53.9, 31.7, 9.2, 7.8 ppm; IR (KBr): 2251, 1701, 1690 cm−1; anal. calcd for C23H21N3O3: C, 71.30; H, 5.46; N, 10.85% found C, 71.22; H, 5.39; N, 10.78%.
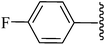
2-[4-Carboethoxy-5-methyl-1-(4-fluorophenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5v). Light yellow solid; yield: 303 mg (75%); m.p.: 194–196 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.55 (s, 1H), 7.46 (s, 1H), 7.35–7.21 (m, 6H), 7.16–7.11 (m, 3H), 4.65 (s, 1H), 4.27–4.20 (m, 2H), 2.32 (s, 3H), 1.39–1.28 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 166.4, 164.0, 159.9, 138.0, 134.8, 133.3, 130.9, 130.7, 129.5, 128.4, 128.3, 117.8, 116.1, 115.8, 111.2, 109.8, 59.4, 37.1, 14.1, 12.4 ppm; IR (KBr): 2250, 1702, 1688 cm−1; anal. calcd for C23H20FN3O3: C, 68.14; H, 4.97; N, 10.36% found C, 68.07; H, 4.91; N, 10.30%.
2-[4-Carboethoxy-5-methyl-1-(4-methoxyphenyl)-2-phenyl-1H-3-pyrrolyl]-2-cyanoacetamide (5w). Light yellow solid; yield: 329 mg (79%); m.p.: 216–218 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.51 (s, 1H), 7.28–7.27 (m, 4H), 7.16–7.14 (m, 4H), 6.92–6.90 (m, 2H), 4.62 (s, 1H), 4.26–4.19 (m, 2H), 3.72 (s, 3H), 2.30 (s, 3H), 1.33–1.28 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 166.4, 164.1, 158.9, 138.1, 134.9, 130.7, 129.8, 129.5, 128.3, 117.8, 114.1, 110.9, 109.5, 59.3, 55.3, 37.1, 14.1, 12.5 ppm; IR (KBr): 2253, 1703, 1693 cm−1; anal. calcd for C24H23N3O4: C, 69.05; H, 5.55; N, 10.07% found C, 68.98; H, 5.49; N, 10.01%.
2-(4-Carboethoxy-5-methyl-1-benzyl-2-phenyl-1H-3-pyrrolyl)-2-cyanoacetamide (5x). Light yellow solid; yield: 293 mg (73%); m.p.: 78–80 °C; 1H NMR (300 MHz, DMSO-d6): δ = 7.51–7.43 (m, 4H), 7.33–7.23 (m, 5H), 7.05 (s, 1H), 7.95 (d, J = 7.2 Hz, 2H), 5.04 (s, 2H), 4.64 (s, 1H), 4.23–4.17 (m, 2H), 2.40 (s, 3H), 1.32–1.24 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 166.2, 164.1, 137.1, 134.3, 130.8, 129.5, 129.1, 128.8, 128.7, 127.2, 125.7, 117.9, 111.2, 109.7, 59.2, 47.3, 36.9, 14.0, 11.5 ppm; IR (KBr): 2248, 1701, 1688 cm−1; anal. calcd for C24H23N3O3: C, 71.80; H, 5.77; N, 10.47% found C, 71.73; H, 5.72; N, 10.41%.
Benzo[g]indole 11; general procedure
The 2-pyrrolyl-2-cyanoacetamide 5 (0.5 mmol) in diphenyl ether (10 mL) was heated at reflux for 10 minutes. Upon completion of the reaction, the crude mass was purified by chromatography on a silica gel column (EtOAc/hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get pure compound 11 as brown solid.
1) to get pure compound 11 as brown solid.
3-Acetyl-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11a). Brown solid; yield: 103 mg (61%); m.p.: 228–230 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.91 (s, 1H), 8.35 (d, J = 8.4 Hz, 1H), 7.73–7.20 (m, 3H), 7.53 (t, J = 3.6 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H), 6.86 (d, J = 8.7 Hz, 1H), 2.62 (s, 3H), 2.28 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.9, 156.4, 140.5, 138.9, 131.0, 130.5, 129.2, 125.4, 124.8, 124.7, 123.2, 120.5, 119.4, 118.0, 117.1, 88.6, 32.6, 12.9 ppm; IR (KBr): 3330, 2214, 1647 cm−1; anal. calcd for C22H16N2O2: C, 77.63; H, 4.74; N, 8.23% found, C, 77.56; H, 4.67; N, 8.16%.
3-Acetyl-5-hydroxy-1-p-tolyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11b). Brown solid; yield: 97 mg (55%); m.p.: 238–240 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.89 (s, 1H), 8.35 (d, J = 8.1 Hz, 1H), 7.53–7.50 (m, 1H), 7.46–7.30 (m, 3H), 7.53 (t, J = 3.6 Hz, 2H), 6.96 (d, J = 8.4 Hz, 1H), 2.61 (s, 3H), 2.50 (s, 3H), 2.26 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.9, 156.4, 140.7, 140.1, 136.2, 131.4, 129.3, 128.9, 125.5, 124.7, 124.5, 123.2, 120.6, 119.3, 117.9, 117.1, 88.6, 32.6, 21.3, 12.9 ppm; IR (KBr): 3334, 2216, 1648 cm−1; anal. calcd for C23H18N2O2: C, 77.95; H, 5.12; N, 7.90% found, C, 77.88; H, 5.05; N, 7.83%.
3-Acetyl-5-hydroxy-1-(4-bromophenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11c). Brown solid; yield: 109 mg (52%); m.p.: 212–214 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.96 (s, 1H), 8.36 (d, J = 7.8 Hz, 1H), 7.94–7.90 (m, 2H), 7.56–7.42 (m, 3H), 7.30–7.14 (m, 1H), 6.95 (d, J = 6.9 Hz, 1H), 2.61 (s, 3H), 2.27 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 196.0, 156.5, 140.5, 138.2, 134.0, 131.5, 130.4, 129.5, 125.3, 124.9, 123.7, 123.2, 120.5, 119.5, 119.0, 118.3, 117.0, 88.5, 32.7, 12.8 ppm; IR (KBr): 3335, 2216, 1645 cm−1; anal. calcd for C22H15BrN2O2: C, 63.02; H, 3.61; N, 6.68% found, C, 62.94; H, 3.53; N, 6.60%.
3-Acetyl-5-hydroxy-1-(4-chlorophenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11d). Brown solid; yield: 100 mg (53%); m.p.: 253–255 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.89 (s, 1H), 8.26 (d, J = 8.1 Hz, 1H), 7.70 (d, J = 8.4 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H),7.40–7.17 (m, 2H), 6.85 (d, J = 8.1 Hz, 1H), 2.41 (s, 3H), 2.18 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 196.0, 156.5, 140.5, 137.8, 135.1, 131.2, 131.0, 129.5, 125.3, 124.8, 124.7, 124.3, 123.2, 120.4, 117.0, 88.6, 32.6, 12.8 ppm; IR (KBr): 3330, 2219, 1653 cm−1; anal. calcd for C22H15ClN2O2: C, 70.50; H, 4.03; N, 7.47% found, C, 70.44; H, 3.96; N, 7.41%.
3-Acetyl-5-hydroxy-1-(4-fluorophenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11e). Brown solid; yield: 91 mg (51%); m.p.: 247–249 °C. 1H NMR (300 MHz, DMSO-d6): δ = 10.82 (s, 1H), 8.25 (d, J = 8.1 Hz, 1H), 7.56–7.43 (m, 4H), 7.38–7.26 (m, 2H), 6.81 (d, J = 8.4 Hz, 1H), 2.51 (s, 3H), 2.17 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 196.0, 156.5, 140.7, 135.1, 131.6, 131.5, 129.5, 124.8, 124.7, 124.4, 123.2, 120.4, 119.4, 118.1, 117.8, 117.0, 88.5, 32.6, 12.8 ppm; IR (KBr): 3326, 2224, 1648 cm−1; anal. calcd for C22H15FN2O2: C, 73.73; H, 4.22; N, 7.82% found, C, 73.65; H, 4.16; N, 7.74%.
3-Acetyl-5-hydroxy-1-(4-methoxyphenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11f). Brown solid; yield: 109 mg (59%); m.p.: 140–142 °C;1H NMR (300 MHz, DMSO-d6): δ = 10.87 (s, 1H), 8.33 (d, J = 8.4 Hz, 1H), 7.45–7.33 (m, 4H), 7.23 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.4 Hz, 1H), 3.90 (s, 3H), 2.60 (s, 3H), 2.26 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.6, 160.2, 156.1, 140.8, 131.0, 130.1, 129.1, 125.4, 124.5, 124.4, 124.3, 122.9, 120.4, 119.0, 117.6, 116.9, 115.8, 88.4, 55.8, 32.4, 12.7 ppm; IR (KBr): 3330, 2223, 1649 cm−1; anal. calcd for C23H18N2O3: C, 74.58; H, 4.90; N, 7.56% found, C, 74.51; H, 4.84; N, 7.51%.
3-Acetyl-5-hydroxy-1-benzyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11g). Brown solid; yield: 106 mg (60%); m.p.: 202–204 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.94 (s, 1H), 8.37 (bs, 1H), 8.14–8.13 (m, 1H), 7.68–7.51 (m, 2H), 7.33–7.27 (m, 3H), 7.05 (bs, 2H), 5.88 (s, 2H), 2.62 (s, 3H), 2.52 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 196.7, 156.1, 140.1, 137.1, 129.8, 129.5, 127.8, 125.9, 124.8, 124.5, 123.2, 121.4, 119.5, 118.0, 117.2, 88.4, 49.4, 33.0, 11.9 ppm; IR (KBr): 3336, 2216, 1647 cm−1; anal. calcd for C23H18N2O2: C, 77.95; H, 5.12; N, 7.90% found, C, 77.87; H, 5.04; N, 7.84%.
3-Acetyl-5-hydroxy-1-cyclopropyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11h). Brown solid; yield: 94 mg (62%); m.p.: 194–196 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.79 (s, 1H), 8.89 (d, J = 8.7 Hz, 1H), 8.37 (d, J = 8.1 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.56 (t, J = 7.5 Hz, 1H), 3.79 (m, 1H), 2.65 (s, 3H), 2.53 (s, 3H), 1.43–1.42 (m, 2H), 0.87 (bs, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.7, 156.0, 142.5, 128.9, 126.0, 124.8, 124.4, 124.3, 123.5, 123.0, 118.8, 117.5, 117.2, 88.5, 32.6, 29.1, 14.4, 12.4 ppm; IR (KBr): 3331, 2218, 1653 cm−1; anal. calcd for C19H16N2O2: C, 74.98; H, 5.30; N, 9.20% found, C, 74.92; H, 5.23; N, 9.14%.
3-Acetyl-7-chloro-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11i). Brown solid; yield: 99 mg (53%); m.p.: 270–272 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.09 (s, 1H), 8.31 (d, J = 2.1 Hz, 1H), 7.72–7.71 (m, 3H), 7.53–7.52 (m, 2H), 7.37–7.33 (dd, J1 = 9.0 Hz, J2 = 2.1 Hz, 1H), 6.81 (d, J = 9.0 Hz, 1H), 2.59 (s, 3H), 2.26 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.8, 155.4, 141.2, 138.5, 131.1, 130.7, 129.5, 129.4, 129.1, 125.2, 124.2, 123.7, 122.7, 122.6, 118.0, 116.6, 88.9, 32.6, 12.9 ppm; IR (KBr): 3327, 2223, 1648 cm−1; anal. calcd for C22H15ClN2O2: C, 70.50; H, 4.03; N, 7.47% found, C, 70.43; H, 3.97; N, 7.40%.
3-Acetyl-7-chloro-5-hydroxy-1-p-tolyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11j). Brown solid; yield: 98 mg (51%); m.p.: 238–240 °C; 1H NMR (300 MHz, DMSO-d6): δ = 8.31 (bs, 1H), 7.50 (d, J = 7.5 Hz, 2H), 7.39 (d, J = 7.8 Hz, 3H), 6.90 (d, J = 9.0 Hz, 1H), 2.58 (s, 3H), 2.48 (s, 3H), 2.24 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.8, 155.3, 141.4, 140.4, 135.9, 131.6, 129.5, 129.4, 128.8, 125.3, 124.2, 123.6, 122.7, 117.9, 116.6, 90.0, 32.5, 21.3, 12.9 ppm; IR (KBr): 3329, 2218, 1651 cm−1; anal. calcd for C23H17ClN2O2: C, 71.04; H, 4.41; N, 7.20% found, C, 70.97; H, 4.34; N, 7.14%.
3-Acetyl-7-chloro-5-hydroxy-1-benzyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11k). Brown solid; yield: 108 mg (56%); m.p.: 178–180 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.18 (bs, 1H), 8.34 (d, J = 2.1 Hz, 1H), 8.13 (d, J = 9.0 Hz, 1H), 7.56–7.52 (J1 = 9.0 Hz, J2 = 2.1 Hz, 1H), 7.36–7.25 (m, 4H), 7.02 (d, J = 7.2 Hz, 1H), 5.87 (s, 2H), 2.61 (s, 3H), 2.53 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 196.6, 155.1, 140.6, 136.8, 129.6, 129.5, 129.3, 127.9, 125.9, 124.4, 124.2, 123.8, 123.7, 122.7, 120.0, 118.0, 116.8, 89.7, 49.3, 33.0, 12.0 ppm; IR (KBr): 3329, 2218, 1651 cm−1; anal. calcd for C23H17ClN2O2: C, 71.04; H, 4.41; N, 7.20% found, C, 70.96; H, 4.32; N, 7.13%.
3-Acetyl-7-chloro-5-hydroxy-1-cyclopropyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11l). Brown solid; yield: 91 mg (54%); m.p.: 208–210 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.94 (s, 1H), 8.90 (d, J = 9.3 Hz, 1H), 8.34 (bs, 1H), 7.73–7.70 (J1 = 9.0 Hz, J2 = 2.1 Hz, 1H), 3.79 (m, 1H), 2.65 (s, 3H), 2.52 (s, 3H), 1.43–1.41 (m, 2H), 0.88 (bs, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.4, 154.7, 142.9, 129.0, 128.6, 125.6, 123.8, 123.0, 122.9, 119.0, 117.3, 116.5, 89.6, 32.3, 28.8, 14.2, 12.1 ppm; IR (KBr): 3338, 2221, 1653 cm−1; anal. calcd for C19H15ClN2O2: C, 67.36; H, 4.46; N, 8.27% found, C, 67.29; H, 4.40; N, 8.21%.
3-Acetyl-7-methoxy-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11q). Brown solid; yield: 96 mg (52%); m.p.: 188–190 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.79 (s, 1H), 7.72 (bs, 4H), 7.52 (bs, 2H), 6.99 (d, J = 8.1 Hz, 1H), 6.77 (d, J = 9.3 Hz, 1H), 3.83 (s, 3H), 2.68 (s, 3H), 2.25 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 195.8, 156.4, 155.4, 139.8, 138.9, 131.0, 130.5, 129.2, 124.5, 122.3, 120.2, 119.3, 117.7, 104.6, 89.0, 55.7, 32.6, 12.9 ppm; IR (KBr): 3330, 2223, 1649 cm−1; anal. calcd for C23H18N2O3: C, 74.58; H, 4.90; N, 7.56% found, C, 74.50; H, 4.83; N, 7.51%.
3-Carboethoxy-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11u). White solid; yield: 110 mg (59%); m.p.: 218–220 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.86 (s, 1H), 8.36 (d, J = 8.1 Hz, 1H), 7.73–7.71 (m, 3H), 7.56–7.53 (m, 2H), 7.44 (t, J = 7.5 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H), 6.86 (d, J = 8.7 Hz, 1H), 4.41–4.34 (m, 2H), 2.32 (s, 3H), 1.39 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 159.0, 150.7, 137.3, 133.0, 125.2, 124.7, 123.4, 123.3, 119.6, 118.9, 118.5, 117.3, 114.7, 113.6, 111.4, 101.2, 83.1, 54.1, 8.8, 6.7 ppm; IR (KBr): 3305, 2214, 1712 cm−1; anal. calcd for C23H18N2O3: C, 74.58; H, 4.90; N, 7.56% found, C, 74.51; H, 4.83; N, 7.50%.
3-Carboethoxy-5-hydroxy-1-(4-fluorophenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11v). White solid; yield: 108 mg (56%); m.p.: 246–248 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.90 (s, 1H), 8.36 (d, J = 7.8 Hz, 1H), 7.67–7.53 (m, 4H), 7.48–7.36 (m, 2H), 6.91 (d, J = 8.1 Hz, 1H), 4.41–4.34 (m, 2H), 2.33 (s, 3H), 1.39 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 164.4, 164.2, 160.9, 156.2, 142.9, 134.7, 131.1, 129.0, 125.1, 124.4, 123.9, 122.8, 120.0, 119.1, 117.4, 116.5, 106.8, 88.5, 59.5, 14.2, 12.1 ppm; IR (KBr): 3302, 2216, 1715 cm−1; anal. calcd for C23H17FN2O3: C, 71.13; H, 4.41; N, 7.21% found, C, 71.06; H, 4.34; N, 7.14%.
3-Carboethoxy-5-hydroxy-1-(4-methoxyphenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11w). White solid; yield: 122 mg (61%); m.p.: 256–258 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.84 (s, 1H), 8.34 (d, J = 8.1 Hz, 1H), 7.47–7.36 (m, 4H), 7.24–7.20 (m, 2H), 7.00–6.97 (m, 1H), 4.40–4.33 (m, 2H), 3.91 (s, 3H), 2.32 (s, 3H), 1.41–1.35 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 164.4, 160.0, 156.1, 143.2, 130.8, 129.8, 128.9, 125.2, 124.3, 124.1, 122.7, 120.1, 118.9, 116.6, 115.6, 106.4, 88.5, 59.4, 55.6, 14.2, 12.1 ppm; IR (KBr): 3301, 2213, 1711 cm−1; anal. calcd for C24H20N2O4: C, 71.99; H, 5.03; N, 7.00% found, C, 71.92; H, 4.97; N, 6.93%.
3-Carboethoxy-5-hydroxy-1-benzyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11x). White solid; yield: 105 mg (55%); m.p.: 192–194 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.85 (s, 1H), 8.37 (d, J = 7.8 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 7.55–7.45 (m, 2H), 7.35–7.22 (m, 3H), 7.02 (d, J = 7.2 Hz, 2H), 5.87 (s, 2H), 4.41–4.34 (m, 2H), 2.61 (s, 3H), 1.39 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 164.6, 155.9, 142.5, 136.8, 129.3, 129.1, 127.4, 125.5, 124.3, 124.2, 123.9, 122.7, 121.0, 119.4, 116.6, 106.5, 88.6, 59.6, 49.1, 14.2, 11.2 ppm; IR (KBr): 3302, 2217, 1710 cm−1; anal. calcd for C24H20N2O3: C, 74.98; H, 5.24; N, 7.29% found, C, 74.91; H, 5.18; N, 7.22%.
3-Benzoyl-5-hydroxy-1-phenyl-2-methyl-1H-benzo[g]indole-4-carbonitrile (11y). Brown solid; yield: 82 mg (41%); m.p.: 256–258 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.78 (s, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 7.2 Hz, 2H), 7.73–7.43 (m, 8H), 7.36 (t, J = 7.8 Hz, 1H), 7.25 (t, J = 6.9 Hz, 1H), 6.86 (d, J = 8.7 Hz, 1H), 1.91 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 191.9, 155.8, 140.3, 139.9, 138.9, 133.4, 130.9, 130.4, 129.9, 129.1, 125.4, 124.8, 124.6, 124.5, 123.2, 120.4, 116.2, 115.1, 88.2, 12.8 ppm; IR (KBr): 3335, 2210, 1649 cm−1; anal. calcd for C27H18N2O2: C, 80.58; H, 4.51; N, 6.96% found, C, 80.51; H, 4.44; N, 6.90%.
3-Benzoyl-5-hydroxy-1-(4-methoxyphenyl)-2-methyl-1H-benzo[g]indole-4-carbonitrile (11z). Brown solid; yield: 93 mg (43%); m.p.: 248–250 °C; 1H NMR (300 MHz, DMSO-d6): δ = 10.83 (s, 1H), 8.34 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 8.1 Hz, 2H) 7.67–7.35 (m, 7H), 7.23 (d, J = 8.7 Hz, 2H), 7.06 (d, J = 8.4 Hz, 1H), 3.90 (s, 3H), 1.98 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 191.8, 160.3, 155.8, 140.7, 139.9, 133.4, 131.3, 130.2, 129.9, 125.5, 124.7, 124.5, 123.2, 120.5, 120.2, 116.3, 115.9, 114.9, 8.2, 55.9, 12.8 ppm; IR (KBr): 3336, 2225, 1647 cm−1; anal. calcd for C28H20N2O3: C, 77.76; H, 4.66; N, 6.48% found, C, 77.70; H, 4.59; N, 6.41%.
2,3-Dicarboethoxy-5-hydroxy-1-aryl-1H-benzo[g]indole-4-carbonitrile 13; general procedure
Dicarboethoxy substituted-2-cyanoacetamide 12 (0.5 mmol) in diphenyl ether (10 mL) was heated at reflux for 10 minutes. Upon completion of the reaction, the crude mass was purified by chromatography on a silica gel column (EtOAc/hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get pure compound 13 as white solid.
1) to get pure compound 13 as white solid.
2,3-Dicarboethoxy-5-hydroxy-1-phenyl-1H-benzo[g]indole-4-carbonitrile (13a). White solid; yield: 137 mg (64%); m.p.: 180–182 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.32 (s, 1H), 8.31 (d, J = 8.4 Hz, 1H), 7.61–7.47 (m, 6H), 7.29 (t, J = 8.4 Hz, 1H), 6.77 (d, J = 8.4 Hz, 1H), 4.33–4.26 (m, 2H), 4.02–3.95 (m, 2H), 1.28 (t, J = 7.2 Hz, 3H), 0.94 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 158.6, 153.8, 151.5, 133.5, 124.7, 124.5, 124.1, 123.3, 122.8, 121.6, 120.8, 119.7, 119.5, 115.7, 111.7, 110.0, 109.7, 80.8, 55.7, 8.6, 8.1 ppm; IR (KBr): 3270, 2227, 1739, 1714 cm−1; anal. calcd for C25H20N2O5: C, 70.08; H, 4.71; N, 6.54% found, C, 70.01; H, 4.65; N, 6.45%.
2,3-Dicarboethoxy-5-hydroxy-1-p-tolyl-1H-benzo[g]indole-4-carbonitrile (13b). White solid; yield: 115 mg (52%); m.p.: 168–170 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.38 (s, 1H), 8.40 (d, J = 8.1 Hz, 1H), 7.57 (t, J = 7.2 Hz, 1H), 7.44–7.39 (m, 5H), 6.98 (d, J = 8.4 Hz, 1H), 4.41–4.34 (m, 2H), 4.11–4.04 (m, 2H), 2.49 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H), 1.06 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 158.6, 153.8, 151.4, 134.2, 130.8, 124.9, 124.1, 123.0, 121.6, 120.8, 119.6, 119.4, 119.1, 115.7, 111.7, 110.0, 109.5, 80.9, 55.7, 55.6, 15.6, 8.6, 8.2 ppm; IR (KBr): 3272, 2225, 1735, 1716 cm−1; anal. calcd for C26H22N2O5: C, 70.58; H, 5.01; N, 6.33% found, C, 70.51; H, 4.94; N, 6.26%.
2,3-Dicarboethoxy-5-hydroxy-1-(4-fluorophenyl)-1H-benzo[g]indole-4-carbonitrile (13c). White solid; yield: 136 mg (61%); m.p.: 238–240 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.43 (s, 1H), 8.42 (d, J = 7.8 Hz, 1H),7.69–7.47 (m, 6H), 6.94 (d, J = 8.1 Hz, 1H), 4.42–4.36 (m, 2H), 4.13–4.01 (m, 2H), 1.37 (t, J = 7.2 Hz, 3H), 1.08 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 158.7, 153.7, 151.5, 129.8, 125.7, 125.6, 124.3, 122.4, 121.8, 120.9, 119.8, 119.5, 119.1, 115.6, 111.7, 111.2, 110.3, 110.0, 80.7, 55.7, 8.6, 8.2 ppm; IR (KBr): 3271, 2226, 1738, 1714 cm−1; anal. calcd for C25H19FN2O5: C, 67.26; H, 4.29; N, 6.28% found, C, 67.20; H, 4.22; N, 6.21%.
2,3-Dicarboethoxy-5-hydroxy-1-(4-chlorophenyl)-1H-benzo[g]indole-4-carbonitrile (13d). White solid; yield: 138 mg (60%); m.p.: 194–196 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.45 (s, 1H), 8.42 (d, J = 8.1 Hz, 1H), 7.73–7.57 (m, 5H), 7.48 (t, J = 7.2 Hz, 1H), 6.98 (d, J = 8.1 Hz, 1H), 4.43–4.36 (m, 2H), 4.14–4.07 (m, 2H), 1.37 (t, J = 7.2 Hz, 3H), 1.08 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 158.7, 153.6, 151.5, 132.5, 129.2, 125.3, 124.5, 122.1, 121.7, 120.9, 119.8, 119.5, 119.0, 115.6, 111.8, 110.6, 110.0, 80.7, 55.8, 8.6, 8.2 ppm; IR (KBr): 3274, 2221, 1739, 1716 cm−1; anal. calcd for C25H19ClN2O5: C, 64.87; H, 4.14; N, 6.05% found, C, 64.81; H, 4.06; N, 5.96%.
2,3-Dicarboethoxy-5-hydroxy-1-(4-methoxyphenyl)-1H-benzo[g]indole-4-carbonitrile (13e). White solid; yield: 144 mg (63%); m.p.: 180–182 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.41 (s, 1H), 8.41 (d, J = 8.1 Hz, 1H), 7.58 (t, J = 7.5 Hz, 1H) 7.48–7.42 (m, 3H), 7.17 (d, J = 9.0 Hz, 2H), 7.02 (d, J = 8.1 Hz, 1H), 4.41–4.34 (m, 2H), 4.12–4.08 (m, 2H), 3.90 (s, 3H), 1.36 (t, J = 6.9 Hz, 3H), 1.07 (t, J = 6.9 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 164.3, 160.4, 159.5, 157.2, 131.5, 130.1, 129.8, 126.4, 125.1, 124.9, 121.4, 115.8, 115.2, 115.0, 86.8, 61.4, 61.3, 55.9, 14.3, 13.9 ppm; IR (KBr): 3276, 2223, 1735, 1719 cm−1; anal. calcd for C26H22N2O6: C, 68.11; H, 4.84; N, 6.11% found, C, 68.05; H, 4.76; N, 6.04%.
2,3-Dicarboethoxy-5-hydroxy-1-(2,4 dimethyl phenyl)-1H-benzo[g]indole-4-carbonitrile (13f). White solid; yield: 125 mg (55%); m.p.: 176–178 °C; 1H NMR (300 MHz, DMSO-d6): δ = 11.42 (s, 1H), 8.42 (d, J = 7.8 Hz, 1H), 7.58 (t, J = 7.2 Hz, 1H), 7.43 (t, J = 7.2 Hz, 1H), 7.35–7.23 (m, 3H), 6.94 (d, J = 8.4 Hz, 1H), 4.42–4.34 (m, 2H), 4.12–4.04 (m, 2H), 2.45 (s, 3H), 1.98 (s, 3H) 1.37 (t, J = 7.2 Hz, 3H), 1.07 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 158.6, 153.8, 151.5, 134.4, 130.6, 130.0, 126.3, 124.5, 122.9, 122.6, 120.9, 119.6, 119.2, 114.8, 111.8, 110.1, 109.5, 81.0, 55.8, 55.7, 15.5, 11.4, 8.6, 8.1 ppm; IR (KBr): 3273, 2224, 1737, 1714 cm−1; anal. calcd for C27H24N2O5: C, 71.04; H, 5.30; N, 6.14% found, C, 70.97; H, 5.23; N, 6.07%.
Acknowledgements
S. M. and A. K. thank CSIR and UGC, New Delhi, India respectively for offering SRF. The financial assistance of CSIR, New Delhi is gratefully acknowledged [Major Research Project, no. 02(0007)/11/EMR-II]. Crystallography was performed at the DST-FIST, India-funded Single Crystal Diffractometer Facility at the Department of Chemistry, University of Calcutta.
References
-
(a) M. Ishikura and K. Yamada, Nat. Prod. Rep., 2009, 26, 803–852 RSC;
(b) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843–868 RSC;
(c) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761–793 RSC.
-
(a) C. Gil and S. Brase, J. Comb. Chem., 2009, 11, 175–197 CrossRef CAS PubMed;
(b) C. R. Donald and C. L. Richard, Tetrahedron Lett., 2009, 50, 4003–4008 CrossRef PubMed;
(c) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC.
-
(a) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2013, 30, 694–752 RSC;
(b) P. Ruiz-Sanchis, S. A. Savina, F. Albericio and M. Alvarez, Chem.–Eur. J., 2011, 17, 1388–1408 CrossRef CAS PubMed.
-
(a) K. Brunner, A. V. Dijken, H. Börner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, J. Am. Chem. Soc., 2004, 126, 6035–6042 CrossRef CAS PubMed;
(b) T. Tsuchimoto, H. Matsubayashi, M. Kaneko, Y. Nagase, T. Miyamura and E. Shirakawa, J. Am. Chem. Soc., 2008, 130, 15823–15835 CrossRef CAS PubMed;
(c) Y. Xing, B. Hu, Q. Yao, P. Lu and Y. Wang, Chem.–Eur. J., 2013, 19, 12788–12793 CrossRef CAS PubMed.
- B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Verber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. H. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfield, J. Med. Chem., 1988, 31, 2235–2246 CrossRef CAS.
-
(a) M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583–7601 RSC;
(b) E. Sanna, L. Martínez, C. Rotger, S. Blasco, J. González, E. García-España and A. Costa, Org. Lett., 2010, 12, 3840–3843 CrossRef CAS PubMed;
(c) S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponic and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195–7227 RSC.
- J. Mao, L. Wang, W. Dou, X. Tang, Y. Yan and W. Liu, Org. Lett., 2007, 9, 4567–4570 CrossRef CAS PubMed.
-
(a) B. F. Matzanke, G. Muller-Matzanke and K. N. Raymond, Iron Carriers and Iron Proteins, VCH Publishers, New York, 1989, vol. 5 Search PubMed;
(b) X. Liu and E. C. Theil, Acc. Chem. Res., 2005, 38, 167 CrossRef CAS PubMed.
-
(a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed;
(b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875–2911 CrossRef CAS PubMed;
(c) D. F. Taber and P. K. Tirunahari, Tetrahedron, 2011, 67, 7195–7210 CrossRef CAS PubMed.
-
(a) M. Borthakur, S. Gogoi, J. Gogoi and R. C. Boruah, Tetrahedron Lett., 2010, 51, 5160–5163 CrossRef CAS PubMed;
(b) N. Kise, S. Isemoto and T. Sakurai, Org. Lett., 2009, 11, 4902–4905 CrossRef CAS PubMed;
(c) M. G. Ferlin, G. Chiarelotto and G. Malesini, J. Heterocycl. Chem., 1989, 26, 245–249 CrossRef CAS PubMed;
(d) G. A. Pinna, M. A. Pirisi, G. E. Grella, L. Gherardini, J. M. Mussinu, G. Paglietti, A. M. Ferrari and G. Rastell, Arch. Pharm. Pharm. Med. Chem., 2001, 334, 337–344 CrossRef CAS;
(e) P. A. Suryavanshi, V. Sridharan and J. C. Menéndez, Org. Biomol. Chem., 2010, 8, 3426–3436 RSC.
-
(a) S. Maity, S. Pathak and A. Pramamik, Eur. J. Org. Chem., 2013, 2479–2485 CrossRef CAS PubMed;
(b) S. Maity and A. Pramamik, Synthesis, 2013, 45, 2853–2860 CrossRef CAS PubMed;
(c) S. Maity, S. Pathak and A. Pramamik, Eur. J. Org. Chem., 2014, 4651–4662 CrossRef CAS PubMed;
(d) S. Pathak, D. Das, A. Kundu, S. Maity, N. Guchhait and A. Pramanik, RSC Adv., 2015, 5, 17308–17318 RSC.
- X. Feng, Q. Wang, W. Lin, G.-L. Dou, Z. B. Huang and D.-Q. Shi, Org. Lett., 2013, 15, 2542–2545 CrossRef CAS PubMed.
- ESI†.
- M. Antoine, M. Gerlach, E. Günther, T. Schuster, M. Czech, I. Seipelt and P. Marchand, Synthesis, 2012, 44, 69–82 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C data of compounds 5, 11 and 13 and crystallographic data for 5j, 11h, 11u. CCDC 1025342, 1025253, 1042549 and 1025252. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra05780a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand = 1
ligand = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has been measured using Benesi–Hildebrand equation and found to be ∼7.97 × 103 M−1.
1) has been measured using Benesi–Hildebrand equation and found to be ∼7.97 × 103 M−1.
















































![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see ESI, Fig. S8†).
1 ratio (see ESI, Fig. S8†).




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get pure compound 11 as brown solid.
1) to get pure compound 11 as brown solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get pure compound 13 as white solid.
1) to get pure compound 13 as white solid.










