Coumarins as privileged scaffold for anti-inflammatory drug development
Abstract
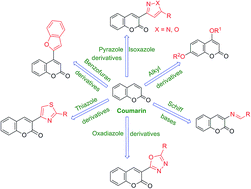
Several literature reports had highlighted the significance of coumarin nucleus as a source of potential candidates for anti-inflammatory drug development. Various natural coumarins such as umbelliferone, scopoletin, visnadin, marmin and esculetin demonstrated potent anti-inflammatory activity through various mechanisms. Keeping in view the importance of naturally occurring coumarins, researchers have extensively explored these by synthesizing various derivatives as anti-inflammatory agents. The present review describes different synthetic modifications carried out on coumarin nucleus, assessment of their anti-inflammatory potential and structure–activity relationship (SAR) study, reported during 1992–2014. SAR may help medicinal chemists in rational design of future anti-inflammatory molecules based on coumarin scaffold.

 Please wait while we load your content...
Please wait while we load your content...