Synthesis of a unique cross-linked polyzwitterion/anion with an aspartic acid residue and its use for Pb2+ removal from aqueous solution†
Abstract
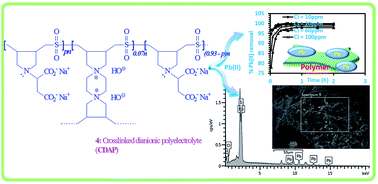
In this work, a unique cross-linked polyzwitterion/anion with an aspartic acid residue was synthesized via butler's cyclopolymerization protocol involving N,N-diallylaspartic acid hydrochloride, 1,1,4,4-tetraallylpiperazinium dichloride and sulfur dioxide in the presence of azoisobutyronitrile. The structure and morphology of the polymer were characterized by using FTIR, TGA, EDX and SEM. The adsorption performance of the resin was evaluated using lead Pb(II) as a model. The effect of various parameters such as contact time, pH, initial concentration and temperature were investigated to arrive at optimum conditions. An optimum pH of 6.0 and dosage of 1.5 g L−1 were obtained. The mechanism of adsorption was investigated using kinetic, diffusion, isotherm and thermodynamic models. The adsorption kinetic data were described well by the pseudo-second order model with R2 of 0.999. The activation energy (Ea) of the adsorption process was calculated as 39.29 kJ mol−1. The negative ΔGo values indicate a spontaneous adsorption process while the negative ΔHo (−43.87 kJ mol−1) suggests an exothermic reaction. Adsorption data were described well by the Langmuir and Temkin models. EDX analysis confirmed the adsorption of Pb2+ on the polymer. The overall results suggest that the polymer could be employed as an efficient adsorbent for the adsorption of toxic Pb2+ from polluted aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...