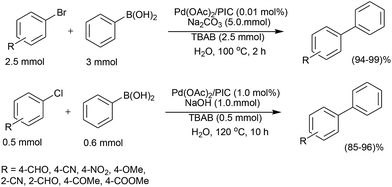
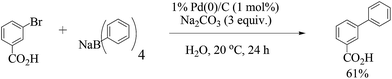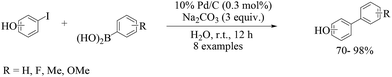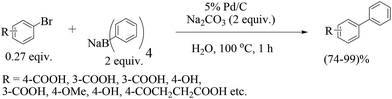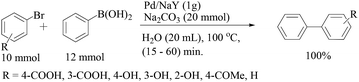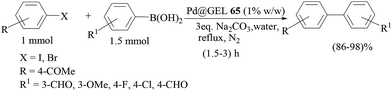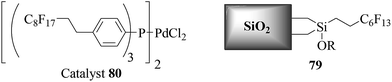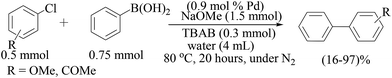Suzuki–Miyaura reaction by heterogeneously supported Pd in water: recent studies
Susmita Paul*a,
Md. Mominul Islamc and
Sk. Manirul Islam*b
aDepartment of Chemistry, University of Kalyani, Nadia, West Bengal, India. E-mail: susmitapaul2007@gmail.com; Fax: +91 33 2582 8282; Tel: +91 94 7419 7728
bDepartment of Chemistry, University of Kalyani, Nadia, West Bengal, India. E-mail: manir65@rediffmail.com; Fax: +91 33 2582 8282; Tel: +91 33 2582 8750
cDepartment of Chemistry, University of Kalyani, Nadia, West Bengal, India
First published on 8th April 2015
Abstract
This review summarizes the progress made essentially in the last fifteen years in the Suzuki–Miyaura coupling reaction by heterogeneous palladium catalysis in water as the sole solvent. The discussion focuses on the heterogenization of the palladium catalyst, efficiency and reusability of the heterogeneous catalysts as well as on the reaction conditions from a sustainable chemistry point of view.
Introduction
For the last few decades, palladium remains the most useful transition metal catalyst in the array of transformations in organic synthesis, in particular for carbon–carbon bond formations.1 The unique nature of the palladium catalyst for selective reactions, easy tuning of the catalyst reactivity and selectivity by ligands or additives, and the high turnover numbers (TONs) and turnover frequencies (TOFs) using extremely small amounts of palladium (ppm or ppb levels) under milder conditions, are the main reasons for attracting researchers, and as a result of these facts a number of palladium catalysts are commercially available2 and employed in many areas, including natural product syntheses.3 Among the different types of palladium-catalyzed reactions, the Suzuki–Miyaura reaction, which is the reaction between aryl halides and arylboronic acids, represents possibly the most important and widely used one.4Suzuki–Miyaura reaction
The Suzuki–Miyaura reaction is characterised by the cross-coupling of two aryl subunits, one from an aryl boronic acid or its derivative and the other from an organohalide or -triflate, to give a biaryl motif.1,5 The relative reactivity order is as follows: R–I > R-OTf > R–Br ≫ R–Cl. This reaction has become one of the most adaptable methods for the expansion of the carbon framework in organic molecules since its discovery in 1979.6 Amongst its wide applicability, the Suzuki–Miyaura reaction is particularly useful as a way of assembling conjugated diene and higher polyene systems of high stereoisomeric purity, as well as biaryl and related systems. Incredible progress has been made in the development of Suzuki–Miyaura coupling reactions of unactivated alkyl halides, enabling C(sp2)–C(sp3) and even C(sp3)–C(sp3) bond-forming processes.1h,i,7 The non-toxicity and simplicity related to the preparation of organoboron compounds (e.g. aryl, vinyl, alkyl),5b,c their relative stability to air and water, combined with relatively mild reaction conditions as well as the formation of nontoxic by-products, makes the Suzuki–Miyaura reaction an important method for enlarging the carbon skeleton.The general and widely accepted mechanism of the Suzuki–Miyaura reaction is depicted in Fig. 1. The first step is the oxidative addition of palladium 1 to halide 2 to form the organopalladium species 3. Reaction of the organopalladium species with a base gives intermediate 4, which via transmetalation with boronate complex 6 forms the organopalladium species 8. Reductive elimination of the desired product 9 restores the original palladium catalyst 1.
Water – a green reaction medium
From the academic as well as industrial viewpoints, alternative reaction media are of considerable concern at present for making this palladium catalyzed cross-coupling process “greener” by minimizing the use of organic solvents.8 Water is the obvious foremost choice in conjunction with cost, environmental benefits, and safety. The use of water in Pd-catalyzed cross coupling reactions dates back to the early development of the Suzuki–Miyaura coupling9 with the first example being reported by Calabrese and co-workers in 1990.10 Since then, a large number of water-soluble Pd catalysts bearing hydrophilic ligands have been reported, and several reviews have been devoted to this subject.11 Attempts have been made for synthesizing water-soluble catalysts or water-soluble ligands,12 adding surfactants or phase-transfer agents,13 using organic co-solvents or inorganic salts as a promoter,14 and utilizing microwave heating or ultrasonic irradiation.15 The use of water as reaction medium would be practically green and welcoming only when any traces of organics or metals can be fully removed from the water used for the reaction.16 For metal catalyzing reactions in which the catalysts are to some extent water soluble, heterogeneous catalysis could solve this issue using a water insoluble support or catalyst which can easily be removed from the medium by filtration. Moreover, for large scale processes, organic products can be separated by simple decantation.Scope and limitations of heterogeneous catalysis
For the synthesis of symmetrical and nonsymmetrical biaryls the palladium-catalyzed carbon–carbon coupling reaction remains an important method, and a broad variety of homogeneous catalytic systems have been developed to achieve this transformation,17 mainly because homogeneous catalysts display high activity and are better defined and understood. Although homogeneous catalysts have many advantages, the complications regarding the separation and recovery of the catalyst, and product contamination with traces of heavy metals, could not be ignored, which limit their applications in chemical and pharmaceutical industries,18 and become an issue of great economic and environmental concern especially for expensive and/or toxic heavy metal complexes.19 These limitations of homogeneous catalysis have resulted in the progress of new strategies for transition-metal catalysis which facilitate catalyst recovery and recycling.20 Recently, many recoverable, supported palladium catalysts have been reported to catalyze Suzuki–Miyaura coupling reactions such as polymers, biomaterials, porous silica, carbon nanotubes, polyurea, natural phosphates etc.21 However, some supported catalysts which are known as heterogeneous catalysts, often resulted in a significant loss of catalytic activity when reused and leaching of transition metal during the reaction,22 and the nature of the true catalyst is still unclear.The problem of distinguishing homogeneous from heterogeneous catalysis is an important question that arises more and more often, in particular when heterogeneous systems are developed. Heterogeneous catalytic systems may partly dissolve to yield a homogeneous component which might be much more reactive than the parent metal surface. For this reason, in some cross-coupling reactions especially with facile substrates catalyzed by trace amounts of metal of various origins, checking is necessary for the genuine source of catalytic metal. Careful kinetic studies, filtration tests, selective poisons for catalysts in solid or soluble systems, and Rebek–Collman 3-phase tests are very helpful and informative to solve the question of heterogeneity.
Aim of the review
The aim of this review is to provide an overview of heterogeneous palladium chemistry for the Suzuki–Miyaura cross-coupling reaction in water as the sole reaction medium or solvent. Since water is genuinely useful for green chemistry as a solvent itself, only protocols carried out in water are covered. One review article23 in this regard by Felpin and co-authors is worth mentioning. The reactions which are not mentioned in that article and the newer reports (up to September, 2014), including all the heterogeneous systems already mentioned by Felpin, are comprised in this review article. The reports consisting of semi-heterogeneous or quasi heterogeneous catalysts, reactions carried out by soluble supports and examples from the patent literature are discarded in this review.Supports are mainly divided into three categories, viz. inorganic, organic and hybrid of inorganic–organic materials, and the discussion is again subdivided according to necessity and for lucidness.
Inorganic supports
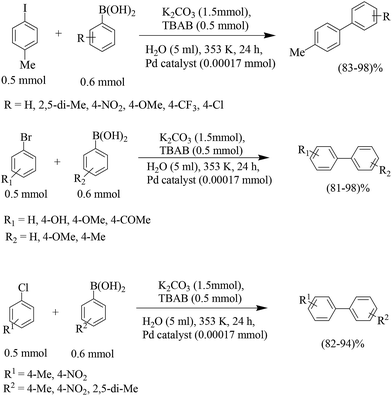
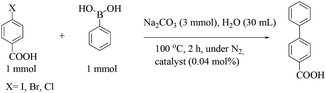
The coupling between iodophenols and boronic acids at room temperature (Scheme 2) could be performed using K2CO3 as the base with a lower loading of Pd/C (0.3 mol%).25 The obtained yields were excellent and fairly independent of the nature of the boronic acid. The reactivity order decreased from iodophenol to bromophenol, and the successful reaction required higher temperatures. After completion of the reaction, the Pd/C catalyst was recovered by simple filtration and reused five times with only a slight decrease in activity.
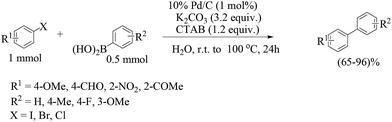
For non-water-soluble aryl halides, a number of reports use surfactants as additives to increase the solubility. Arcadi and co-workers used cetyltrimethylammonium bromide (CTAB) for this purpose, which was found to be quite effective when combined with K2CO3 with a catalyst loading (Pd/C) of 5 mol% (Scheme 3).26 Recycling of the filtered and used catalyst suffered from gradually diminished activity which raises the question of true heterogeneity.
Xu and co-workers27 (Scheme 4) established that water-soluble bromoarenes react efficiently with sodium tetraphenylborate in refluxing water even in the presence of 0.0025 mol% of Pd/C when the reaction time was prolonged from 1 to 7 h. Comparison of the inorganic bases utilized showed the suitability of sodium bases over potassium ones. The reusability of the catalyst showed capability over five cycles but with gradual loss of reactivity (Fig. 2).
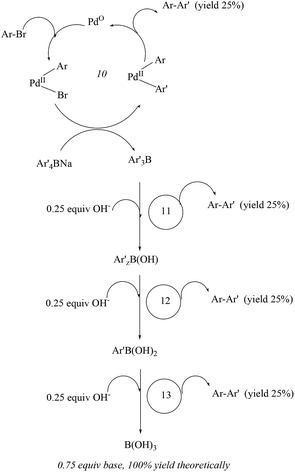 | ||
| Fig. 2 Proposed mechanism of the Pd/C-catalysed cross-coupling of aryl bromide with sodium tetraarylborate by Xu and co-workers. | ||
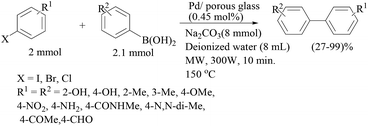
Coupling of aryl chlorides with aryl boronic acids using ligandless Pd/C in water has been described by Kohler and Lysen (Scheme 5).28 All reactions were performed under an ambient atmosphere to reduce homocoupling. Activated aryl chlorides reacted at lower palladium concentrations (0.2–0.5 mol%) while deactivated chloroarenes required higher catalyst concentrations (2.0 mol%) and longer reaction times (six hours). Addition of TBAB was found to be essential, and NaOH was found to be the superior base among the several bases tested. Aryl iodides and bromides could also be completely converted to the corresponding biaryls by a small variation of the reaction conditions. Recovery of the catalyst was performed by simple filtration through celite or by centrifugation. Not only boronic acids but also boronate esters and potassium trifluoroborate salts were effective under the reaction conditions developed by these authors. The reactivation using iodine as the oxidizing agent [Pd(0) to Pd(II)] was necessary to improve the recycling ability of the catalyst, showing consistent activity over three cycles.
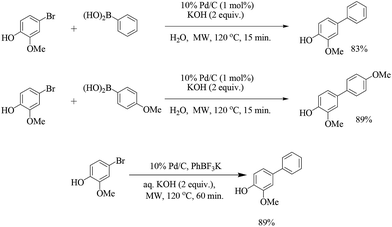
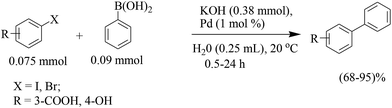
The beneficial effect of microwave heating was explored by Freundlich and Landis (Scheme 6)29 for the coupling of boronic acids with bromophenols. A variety of boronic acids were coupled at 120 °C in aqueous potassium hydroxide for short reaction times (15 min). The reactions remained unsuccessful with the chloro substituent. The coupling of substituted bromophenol with potassium phenyl trifluoroborate salt was less efficient than with phenyl boronic acid under the optimized reaction conditions.
Arvela and Leadbeater reported a combined TBAB and microwave activation procedure for the cross-coupling of aryl chlorides with boronic acids (Scheme 7).30 For substrates bearing electron-withdrawing groups, the effects of simultaneous cooling on the product yield were not significant, attributed to the fact that the coupling reaction is faster than the decomposition of the chloride substrate, but with substrates bearing electron-neutral or electron-donating substituents, simultaneous cooling significantly increased the product yield. Very fast reaction rates were observed in only ten minutes and the method was found to be efficient for aryl chlorides containing electron-withdrawing groups.
The coupling of bromoarenes with tetraphenylborate (Scheme 8) has been described by Bai using a similar catalytic system.31 Excellent yields were obtained in less than twenty minutes at 120 °C in the presence of K2CO3 as the base with a comparatively higher palladium loading (5 mol% Pd). The catalyst showed excellent recycling ability over more than five cycles.
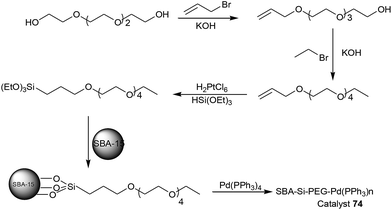
Modified multi-walled carbon nanotubes have been proposed as a support for palladium nanoparticles for the cross-coupling in neat water.32 4-Dimethylaminopyridine (DMAP) stabilized palladium nanoparticles were prepared by mixing solutions of Na2PdCl4 and DMAP followed by the reduction with NaBH4. Thiol-modified multi-walled carbon nanotubes (MWCNTs), prepared by a carbon arc discharge method, were functionalized via an amide coupling reaction followed by sequential treatment with HNO3, KMnO4, HClO4, citric acid, DMAP and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC) and 2-mercaptoethylamine hydrochloride (Fig. 3). Multi-walled carbon nanotube/DMAP-stabilized Pd nanoparticle composites (MWCNT/Pd–DMAP NP composites, catalyst 14) were prepared by the addition of a known amount of a DMAP-stabilized palladium nanoparticle dispersion to thiol-modified multi-walled carbon nanotubes under sonication. The 4-iodo-substituent gave 87% conversion within 10 min with 0.004 mol% of catalyst 14, while the bromo-substituent gave 53% conversion and the chloro-analogue gave 25% conversion with 0.024 mol% of catalyst 14 when refluxing for 6 h. The catalyst was recovered by filtration through a polycarbonate filter, and experimental studies showed good recyclability over six runs with leaching of the Pd species below the detection limit of AAS (Scheme 9).
 | ||
| Scheme 9 Suzuki–Miyaura reaction of interest, using the MWCNT/Pd–DMAP NP composites, between phenylboronic acid and 4-halobenzoic acids. | ||
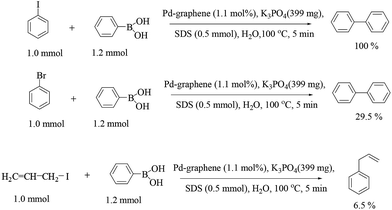
Graphene modified with palladium nanoparticles by reducing palladium acetate [Pd(OAc)2] in the presence of sodium dodecyl sulfate (SDS) was reported by Zhang and co-workers (Scheme 10)33 (SDS is used as both surfactant and the reducing agent). The palladium nanoparticle–graphene hybrids (Pd–graphene hybrids, catalyst 15) were characterized by spectrometric methods, and HRTEM showed that the mean size of the Pd nanoparticles dispersed on the graphene sheets is about 4 nm. Catalyst 15 acted as an efficient catalyst for the Suzuki–Miyaura reaction under aqueous and aerobic conditions, with the reaction reaching completion within 5 min. Bromobenzene and allyl iodides were also employed in this coupling reaction but produced poor yields. The catalysts were recovered by simple centrifugation and reused successfully for ten consecutive runs.
Highly basic nanocrystalline magnesium oxide (NAP-MgO) as a palladium nanoparticle support has been exploited for the Suzuki–Miyaura coupling reaction (Scheme 12).35 Fig. 4 shows the schematic presentation for the preparation of Pd–NAP-MgO (catalyst 17). The cross-couplings of iodo- and bromoarenes with arylboronic acids were efficiently carried out in water, but the reactions involving chloroarenes were performed in DMA with catalyst 17. The cross-couplings were completed in only 5 to 6 h at room temperature at a quite low loading (0.5 mol%); an even lower loading as low as 0.01 mol% is also effective with longer reaction times (40 h). It is supposed that the high activity of the catalyst is due to the nanostructured MgO material that possesses a high surface area (∼600 m2 g−1) and a strong basicity. The catalyst was recyclable for all reactions up to five cycles with almost consistent activity.
Artok and co-workers prepared a highly active catalyst (catalyst 18) by loading NaY zeolite with Pd(NH3)4Cl2 (Scheme 13)36 (NaY zeolite (SiO2/Al2O3 molar ratio: 5.1)) by ion exchange, which gave good yields for the corresponding biphenyl compounds by cross-coupling soluble and insoluble aryl bromides with benzeneboronic acid with catalyst loadings of (0.01–0.001) mol% in water. Electron-rich bromoarenes were found to be much less reactive and required the use of surfactants such as TBAB or CTAB for better results. A possible instability of the catalytic system under localized heating was established by performing the reaction under microwave heating.
The Pd/ZrO2 nanocatalyst formed by electrochemical impregnation of nanostructured tetragonal ZrO2 with palladium nanoparticles (PdNPs/ZrO2, catalyst 19) was demonstrated to be a very efficient catalyst in Suzuki–Miyaura reactions of aryl halides in water, by Nicola Cioffi and co-workers (Scheme 14).37 The catalyst efficiency was attributed to the stabilization of Pd nanophases provided by tetra alkyl ammonium hydroxide, which behaved both as a base as well as a PTC (phase transfer catalyst) agent. The Suzuki–Miyaura cross-coupling reactions were carried out in water at 90 °C using aryl bromides and iodides as substrates and phenyl boronic acids. The supported catalyst could be recycled up to ten times without any appreciable loss of activity which was supported by an average yield of 83% for a number of aryl bromides, except for the less reactive electron-rich 4-bromoanisole.
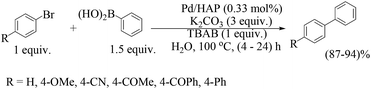
Two types of these supported palladium catalyst, one by immobilization of [Pd(COD)Cl2] (COD = 1,5-cyclooctadiene) on hydroxyapatite (catalyst 21) and another catalyst by subsequent reduction of the previous catalyst with sodium borohydride (catalyst 22), were prepared (Fig. 5) for the Suzuki–Miyaura coupling reaction in water.39 The catalyst with Pd2+, was found to be almost five times more active than the reduced catalyst under similar reaction conditions. The best catalytic activities were observed in the presence of potassium carbonate as the base and tetrabutylammonium bromide as a promoter using the non-reduced catalyst and water as the solvent under aerobic conditions (Scheme 16). This catalyst system has been tested for different electronically neutral, electron-rich, electron-poor and sterically hindered aryl boronic acids, and several different aryl halides including aryl chlorides. More than one thousand turnovers and high selectivities toward the hetero-coupled products have been observed in most cases. A negligible drop in activity was observed over ten cycles.
 | ||
| Fig. 6 Schematic outline of the synthesis of catalyst 22 from pore-expanded MCM-41 and supported monodispersed Pd nanoparticles. | ||
Organic support
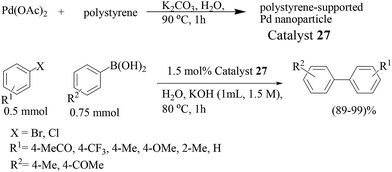
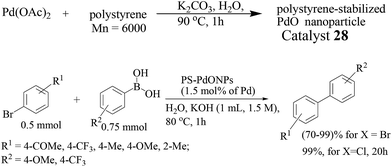
Palladium nanoparticles stabilized onto linear polystyrene (catalyst 27) by thermal decomposition of Pd(OAc)2 was examined in the Suzuki–Miyaura reaction in 1.5 M aqueous KOH solution (Scheme 22).45 A fairly uniform particle size of 2.3 ± 0.3 nm was obtained and ICP-AES revealed that the catalyst contained an average of 2.5 mmol g−1 of Pd. The immobilization degree of palladium was dependent on the molecular weight of polystyrene, while the size of the nanoparticles was not. The cross-coupling reaction of bromobenzene with p-methylphenylboronic acid proceeded efficiently to give 4-methylbiphenyl in 99% yield. Both electron-rich and electron-deficient aryl bromides were reactive under these reaction conditions, affording the desired coupling products in high yields. The catalyst could be recovered by simple filtration. The average yield of 4-methylbiphenyl from the 1st through to the 10th recovered catalysts was 99%. No leaching of palladium into the solution during the reaction was observed by ICP-AES. Worth mentioning is that the reaction proceeded well with aryl chloride and the catalyst could even be recycled.
In a similar study, linear polystyrene-stabilized PdO nanoparticles (PS–PdONPs, catalyst 28) were prepared in water by thermal decomposition of Pd(OAc)2 in the presence of polystyrene, and the Pd nanoparticles (PS–PdNPs) were also prepared using NaBH4 and phenylboronic acid as reductants.46 The catalytic activity of PS–PdONPs was found slightly higher than that of PS–PdNPs for the Suzuki–Miyaura coupling reaction in water probably due to the presence of oxygen and the size effect. The TEM image of catalyst 28 showed a uniform particle size of 2.3 ± 0.3 nm. Under optimized conditions, the Suzuki–Miyaura coupling reaction of bromobenzene with 4-methylphenylboronic acid in 1.5 M KOH aqueous solution at 80 °C for 1 h proceeded efficiently to give 4-methylbiphenyl in 99% yield (Scheme 23). Both electron-rich and electron-deficient aryl bromides were reactive, affording the desired coupling products in high yields. However, the reaction of chlorobenzene gave a lower yield. The catalyst was recovered by filtration and was recycled for 10 times without any loss of activity. No leaching of palladium into the reaction occurred during the reaction, as confirmed by ICP-AES.
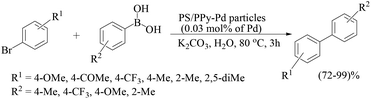
Polypyrrole–palladium nanocomposite-coated cross-linked polystyrene latex particles (PS/PPy–Pd, catalyst 29) have been applied with an excellent catalytic activity to the Suzuki–Miyaura coupling reaction in water (Scheme 24).47 The catalyst was prepared by adding an aqueous solution of PdCl2 and NaCl to a premixed aqueous dispersion of pyrrole and polystyrene. The polymerization was allowed to proceed for 7 days at 200 rpm. The PS/PPy–Pd particles were subsequently purified by repeated centrifugation–redispersion cycles followed by freeze-drying overnight. The potency of the PS/PPy–Pd particles as a catalyst with low metal loading (0.03 mol% of Pd) was examined in the Suzuki–Miyaura coupling reaction of various aryl halides with arylboronic acids in 1.5 mol L−1 aqueous potassium carbonate solution as test reactions. Steric hindrance did not matter as was observed from the high yield of 2,4-o-dimethylbiphenyl from the coupling reaction of 2-bromotoluene with 4-methylphenylboronic acid. ICP-AES analyses confirmed that both the aqueous phase and the organic phase contained barely detectable levels of palladium and that the Pd loading in the particles did not change even after the fifth run, which indicated that no/little Pd nanoparticles detached from the PS/PPy–Pd particles.
An amphiphilic resin-supported triarylphosphine–palladium complex bound to a polyethylene glycol–polystyrene graft copolymer (PEG–PS resin) has been described for the cross-coupling of aryl iodides with boronic acids using KOH as the base.49 The PEG–PS resin-supported palladium–monophosphine complex Pd–PEP (32) was readily prepared by treatment of the resin-supported phosphine (31) with an excess amount of di(μ-chloro)bis(η-allyl)dipalladium(II) ([PdCl(η3-C3H5)]2) (Pd/P > 1/1) followed by the removal of not immobilized [PdCl-(η3-C3H5)]2 by washing with chloroform (Scheme 26).
 | ||
| Scheme 26 Schematic diagram for the synthesis of the resin-supported triarylphosphine–palladium complex. | ||
This catalytic system was found to be more active than the comparable usual homogeneous palladium–phosphine complexes under the same reaction conditions (Scheme 27). No examples with chloroarenes were reported but good yields were obtained with aryl iodides and bromides under mild conditions (25 °C).
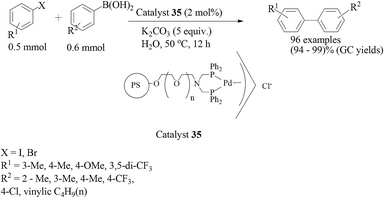
In another report, another resin-supported palladium catalyst (PS–PEG-adppp) for the Suzuki–Miyaura coupling reaction in water has been described by Uozumi and co-workers by merely changing the ligand.50 The catalyst 35 was prepared by treatment of PSPEG–NH2 with diphenylphosphinomethanol (Scheme 28) in toluene–MeOH at 25 °C for 3 h to give PS–PEG-adppp with a quantitative loading value of 0.32 mmol g−1. The palladium complex of the bisphosphine ligand PS–PEG-adppp was prepared by mixing [PdCl(η3-C3H5)]2 in toluene at 25 °C for 15 min to give [PS–PEG-adppp-Pd-(η3-C3H5)]Cl (catalyst 35) in a quantitative yield. Altogether, ninety six combinations of eight aromatic halides and twelve different boronic acids were reported from which a clear idea about the extremely efficient and stable heterogeneous catalyst 35 could be obtained. The reactions were carried out in aqueous K2CO3 at 85 °C (Scheme 29). Catalyst 35 can be recovered by simple filtration and reused without any loss of activity.
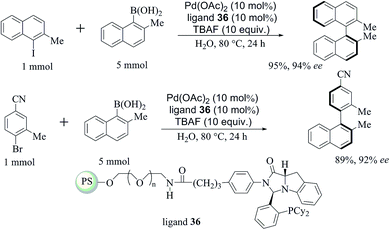
A similar strategy was followed by the same author to introduce the asymmetric Suzuki–Miyaura cross-coupling reaction,51 and achieved by anchoring chiral imidazoindolephosphine to an amphiphilic polystyrene–polyethylene glycol copolymer (PS–PEG) resin (Scheme 30). Excellent yields with very good enantioselectivities (88–99% ee) were obtained at room temperature in water with the aid of a large excess of TBAF (10 equiv.) and boronic acid (5 equiv.). A large amount (10 mol%) of the palladium catalyst was required, but it could be reused after simple filtration with consistent results.
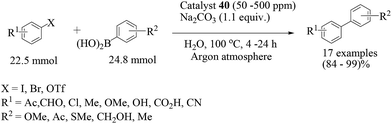
Ikegami and co-workers designed a self-assembled complex of palladium and a non-crosslinked amphiphilic polymer supported through phosphines (Scheme 31).52 The catalyst support was prepared by random polymerization of 4-diphenylstyrylphosphine (37) with 12 equiv. of N-isopropylacrylamide (38) in the presence of 4 mol% AIBN, which gave 39 in 89% yield. Catalyst 40 was prepared by self-assembly of 39 and (NH4)2PdCl4. The catalytic activity of this palladium-network catalyst 40 has been investigated for Suzuki–Miyaura reaction in refluxing water where Na2CO3 was the ultimate choice as a base (Scheme 32). The protocol allowed the reaction of aryl bromides and aryl iodides as substrates, but aryl triflates remained unaffected.
Only trace amounts of the highly active palladium-network complex (50–500 ppm) were required for a successful reaction. The catalyst was found to achieve the coupling of unusual alkenyl halides and alkenylboronic acids at a low catalyst concentration (500 ppm). The recyclability of catalyst 40 was examined for the preparation of biphenyl for up to ten consecutive cycles with a consistent activity.
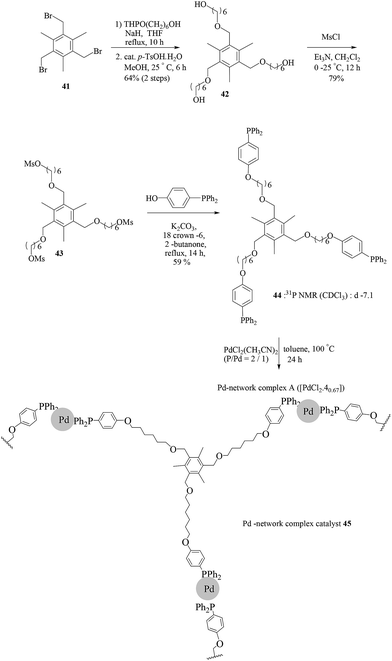
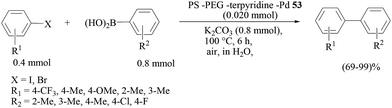
Uozumi and co-workers further developed the idea of a novel palladium complex embedded in a three-dimensional network complex (Scheme 33).53 A novel 3D palladium-network complex catalyst 45 was obtained by self-assembly of PdCl2 and C3-trisphosphine 44, which was prepared from the commercially available 2,4,6-tris-(bromomethyl)mesitylene (41) in four steps. Catalyst 45 showed a high catalytic efficiency at a low loading of 0.05 mol% palladium, and twenty seven examples of the Suzuki–Miyaura reaction in refluxing water with a variety of bromo- and iodoarenes were reported (Scheme 34). Finally, the catalytic complex displayed reusability properties over four successive cycles.
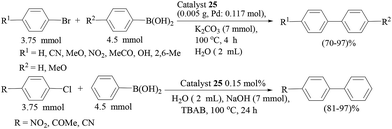
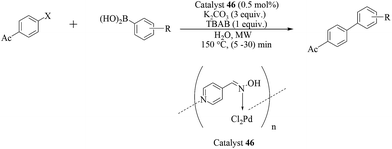
Following their earlier studies, Kirschning et al. considered the aqueous Suzuki–Miyaura reactions of another closely related catalytic system prepared from a 2-pyridine aldoxime-based Pd(II) complex covalently anchored onto a glass–polymer composite material (catalyst 47).55 Aryl and heteroaryl bromides were efficiently coupled with boronic acids at fairly low palladium loadings (0.7 mol%) with the aid of TBAB as the surfactant and KOH as the base, under both thermal (100 °C) or microwave heating (160 °C) conditions (Scheme 36). For chloroarenes, only the coupling of 4-chloroacetophenone has been reported. The catalyst could be reused at least seven times with consistent activity regardless of the source of heating.
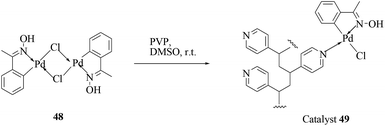
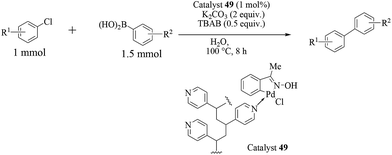
In another report following the previous one, Kirschning and co-workers explored an alternative approach for immobilization of an oxime carbapalladacycle (48) onto polyvinylpyridine as the support (Scheme 37).56 The polymeric phase was prepared from a heated solution (70 °C) of the monomers vinylpyridine and divinylbenzene with AIBN in a nonpolar solvent. While aryl chlorides were more efficiently coupled in water, aryl bromides were preferentially reacted with boronic acids in toluene under these catalytic conditions (Scheme 38). The protocol is associated with the use of TBAB (0.5 equiv.) and microwave activation. The protocol was equally applicable as a thermal, microwave or continuous flow method.
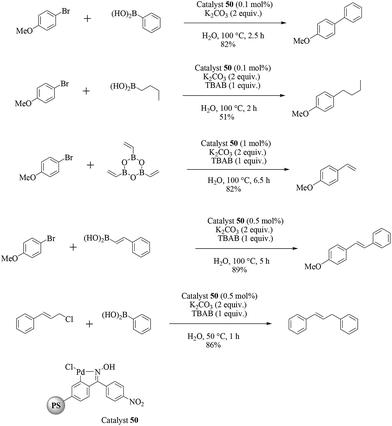
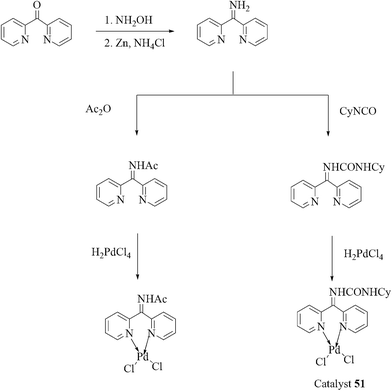
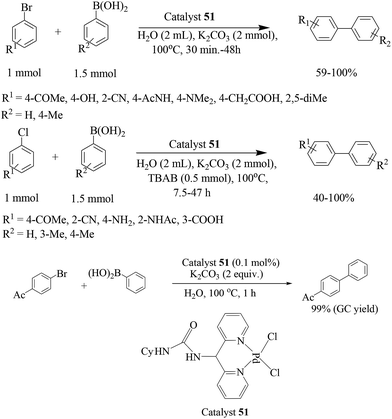
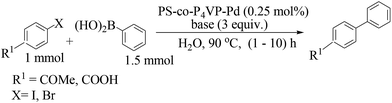
Taking advantage of a rich experience in oxime carbapalladacycle catalysts for cross-coupling reactions in organic and aqueous media,57 Najera and co-workers prepared the palladated Kaiser oxime resin catalyst 50 as an active precatalyst for different types of the Suzuki–Miyaura reaction (Scheme 39).58 Several examples were investigated involving the cross-coupling of aryl bromides, and allyl and benzyl chlorides with aryl-, alkyl- and alkenylboronic acids in neat water. Aryl bromides were efficiently cross-coupled with benzeneboronic acid, but aryl chlorides were poorly reactive. Alkylboronic acid, trivinylboroxine and trimethylboroxine reacted with aryl bromides in the presence of TBAB as an additive. Analysis of the solution showed moderate metal leaching which allowed the reuse of the recovered catalyst 50 with a gradually decreased catalytic activity.
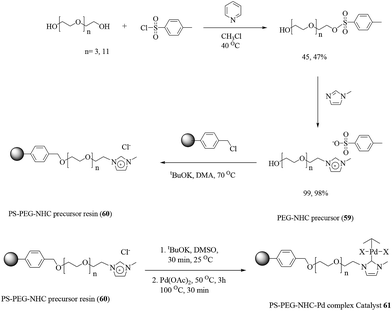
Palladium nanoparticles fixed in the layer of core–shell poly(styrene-co-4-vinylpyridine) microspheres (catalyst 52) were found to be catalytically active for Suzuki–Miyaura cross-couplings in water (Scheme 42).61 The supported catalyst was prepared by adding an aqueous solution of PdCl2 into the colloidal dispersion of the core–shell PS-co-P4VP microspheres at room temperature followed by the dropwise addition of excess NaBH4 aqueous solution. The resultant colloidal dispersion was purified by dialyzing against water at room temperature for 4 days. Transmission electron microscopy (TEM) analyses evidenced that the palladium nanoparticles were uniformly distributed with an average size of 4.4 nm on the polyvinylpyridine shell. Optimization studies revealed that hydrophobic reagents were best coupled with Et3N as a base, while hydrophilic substrates preferably required K2CO3. The catalytic system was examined for the coupling of benzeneboronic acid with a range of unchallenging bromo- and iodoarenes. Chloroarenes, however, were almost unreactive under the same reaction conditions. Recycling studies carried out for the coupling of 4-bromoacetophenone with benzeneboronic acid showed 99% yield of the targeted biaryl compound throughout five consecutive runs. The average size of the palladium nanoparticles remained the same during recycling.
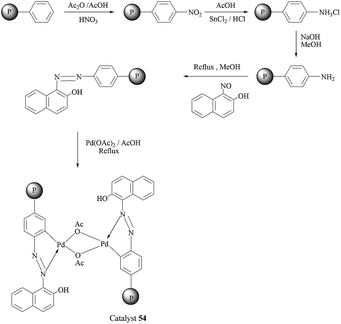
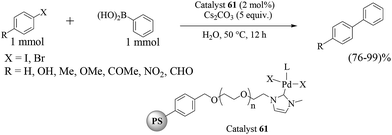
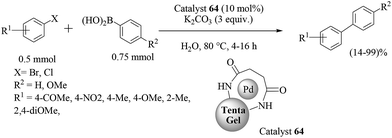
A novel heterogeneous transition-metal catalyst comprising a polymer-supported terpyridine palladium(II) complex (catalyst 53) was prepared (Scheme 43) and found to promote the Suzuki–Miyaura reaction in water under aerobic conditions with high to excellent yields.62,63 The Suzuki–Miyaura cross-coupling reaction of iodobenzene with phenylboronic acid was carried out with K2CO3 (2 equiv.) in the presence of polymeric catalyst 53 (5 mol% Pd) in water to give biphenyl in 93% yield (Scheme 44). A variety of boronic acids and halo arenes with different types of substitution at different positions showed almost excellent yields, thereby proving that the substrate or reactant structures do not affect the reaction yield. The catalyst was recovered by simple filtration and directly reused several times without loss of catalytic activity (ICP-AES analysis (detection limit of Pd: <3 μg L−1) from aqueous or organic filtrates).63
A set of three new polymer-anchored palladium(II) Schiff base catalysts have been synthesized (Scheme 46), characterized and their catalytic activity was investigated in the Suzuki–Miyaura cross-coupling reaction between aryl halides and arylboronic acids in the presence of Cs2CO3 as the base.65 They showed excellent catalytic activity in the coupling of aryl bromides or aryl iodides with phenylboronic acid under the optimized reaction conditions in water (Scheme 47). The polymer-anchored Pd(II) complexes provided turnover frequencies of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 58
700 and 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 h−1 in the Suzuki–Miyaura coupling reactions of phenylboronic acid with p-bromo acetophenone and p-iodobenzene, respectively, which are the highest values ever reported for the Suzuki–Miyaura coupling reaction in water as the sole solvent. The highest conversion reached was up to 45% in the presence of Cs2CO3 within 30 min in water at 100 °C, and longer reaction times did not yield any further conversion for aryl chlorides and phenyl boronic acids. Catalyst 55 maintained 97% of its initial catalytic activity at the end of the fifteen cycle.
200 h−1 in the Suzuki–Miyaura coupling reactions of phenylboronic acid with p-bromo acetophenone and p-iodobenzene, respectively, which are the highest values ever reported for the Suzuki–Miyaura coupling reaction in water as the sole solvent. The highest conversion reached was up to 45% in the presence of Cs2CO3 within 30 min in water at 100 °C, and longer reaction times did not yield any further conversion for aryl chlorides and phenyl boronic acids. Catalyst 55 maintained 97% of its initial catalytic activity at the end of the fifteen cycle.
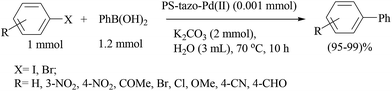
 | ||
| Scheme 48 Triazole-functionalized polystyrene resin-supported Pd(II) [PS-tazo-Pd(II)] complex (catalyst 58). | ||
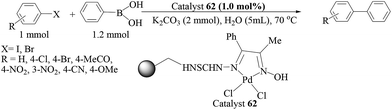
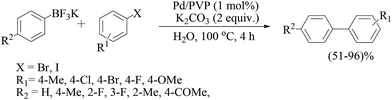
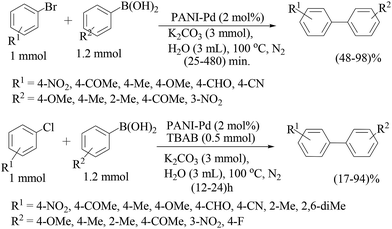
Another report of polyaniline-supported palladium was by M. L. Kantam and co-workers.75 Four types of catalyst were prepared from different palladium precursors and all the catalysts were tested in Suzuki–Miyaura couplings of bromo- and chloroarenes in water (Scheme 60), and the catalyst prepared from the PdCl2 precursor was found to be the most effective catalyst. Cs2CO3 was found to be the best one though other inorganic bases like K3PO4 and KF were also found to be fairly active under these conditions in comparison to organic bases like Et3N and Bu3N. Bromoarenes with electron-withdrawing functionalities reacted at a faster rate than the bromoarenes bearing electron-donating functionalities. Arylboronic acids with an electron-withdrawing group required longer times than those with an electron-donating group. Sterically hindered arylbromides as well as arylboronic acids reacted sluggishly. The catalyst loading could be reduced to 0.5 and 0.1 mol% but longer reaction times were required for excellent yields. The catalyst was used for five consecutive cycles and the difference in palladium content between the fresh and used catalyst was ca. 2%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 mmol of NIPA
1.6 mmol of NIPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMA exhibited optimum catalytic activity, and could effectively be reused 5–6 times without loss of catalytic activity. The study revealed that the hydrophilicity of the catalyst and its swelling in the water medium played a decisive role in determining the catalytic performance.
PMA exhibited optimum catalytic activity, and could effectively be reused 5–6 times without loss of catalytic activity. The study revealed that the hydrophilicity of the catalyst and its swelling in the water medium played a decisive role in determining the catalytic performance.
 | ||
| Scheme 61 Schematic representation of the synthesis process of the Pd@poly(NIPA-co-PMA) hydrogel catalyst. | ||
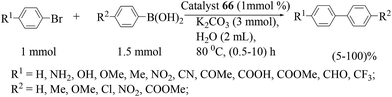
Palladium nanoparticles on a hydrogel (catalyst 66) prepared by Rhee and co-workers (Scheme 63)77 showed high activity in Suzuki–Miyaura coupling reactions of arylboronic acids and aryl bromides with a wide range of functional groups, containing electron-donating and electron-withdrawing groups, in water and under mild reaction conditions (80 °C) (Scheme 64). The catalyst showed excellent activity for the first five runs, resulting in high yields above 90–95% in 40 min. After each run, the amount of Pd leaching was estimated by performing ICP measurements on the supernatant solutions. No significant Pd leaching (<0.5 ppm) was observed.
 | ||
| Scheme 63 (a) Preparation of the PNIPAM-co-4-VP co-polymers, and (b) preparation of Pd nanoparticles on the PNIPAM-co-4-VP co-polymer. | ||
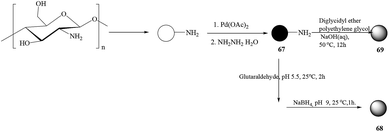
Following the previous report, a chitosan-g-mTEG (methoxy triethylene glycol)- or -mPEG (methoxy polyethylene glycol)-supported palladium(0) catalyst (Scheme 67) for the Suzuki–Miyaura cross-coupling reaction in water has been demonstrated by the same author (Scheme 68).79 The catalyst showed excellent catalytic activity in the Suzuki–Miyaura cross-coupling reaction without additional phase-transfer reagents due to the enhanced solubility of the organic substrate by PEG grafting. In addition, the catalyst could be reused up to five times with the catalytic activity being recovered easily after simple manipulations.
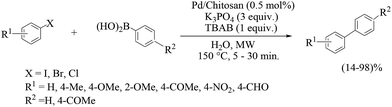
The activity of the g-mTEG or -mPEG Pd(0) catalyst was similar to the one of the CS–Pd(0) catalyst when TBAB was used as a phase-transfer reagent, but without TBAB, the activity of the CS-g-mPEG Pd(0) catalyst was superior to that of the CS–Pd(0) catalyst which demonstrated that grafted mTEG or mPEG worked as an effective phase-transfer reagent in the Suzuki–Miyaura cross-coupling reaction in water. Aryl iodides and bromides gave viable product yields while aryl chlorides gave comparatively lower yields. CS-g-mPEG Pd(0) showed better catalytic activity than the non-grafted CS–Pd(0) catalyst and was found to be effective for the coupling of aryl chloride in water. The CS-g-mPEGPd(0) catalyst was reused up to five times with gradually decreasing activity which might be due to the aggregation of palladium nanoparticles inside the chitosan beads, according to the authors.
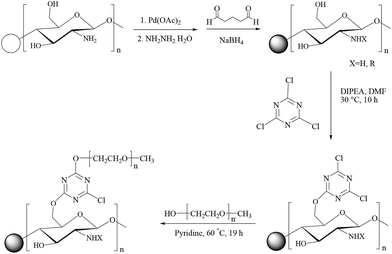
Another report on chitosan-supported Palladium catalysts (Scheme 69) is by Pombeiro et al.80 Pd-Chit 70 and Pd-Chit 71 (Fig. 7) have been exploited for model microwave-assisted Suzuki–Miyaura cross-coupling reactions in water to give excellent yields. The effects of catalyst loading, temperature, time, the phase-transfer agent tetrabutylammonium bromide (TBAB) and base were investigated. The catalytic reactions of the supported material proceeds heterogeneously as proven by activity studies and ICP-AES analyses on leaching. Although the presence of the phase-transfer agent TBAB favoured the reaction, its effect was less pronounced with the increase in temperature and become negligible at 160 °C. The presence of a base was fundamental and potassium carbonate was the best one among those screened. The chitosan-supported heterogeneous catalyst could be successfully recovered and reused up to seven times though with a gradual loss of catalytic activity.
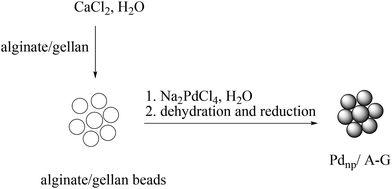
The use of palladium nanoparticles stabilized by natural beads made of an alginate/gellan mixture (Fig. 8, Scheme 70) in the Suzuki–Miyaura cross-coupling reaction of arenediazonium tetrafluoroborates with potassium aryltrifluoroborates (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) with a catalyst loading as low as 0.01–0.002 mol% under aerobic, and phosphine- and base-free conditions in water is described (Scheme 71).81 Good to high yields were obtained with arenediazonium tetrafluoroborates and potassium aryltrifluoroborates containing both electron-donating and electron-withdrawing substituents. However, a more severe steric congestion leads to trace amounts of the desired product. SEM analysis, at higher magnification, displayed aggregated nanoparticles and TEM measurements indicated that the catalyst system before use contained spheroidal palladium nanoparticles, whose average size was 3.4 ± 1.4 nm. The catalyst system could be reused several times without significant loss of activity. The material recovered after eight runs showed nanoparticles of about 3.6 ± 1.7 nm in diameter.
1 molar ratio) with a catalyst loading as low as 0.01–0.002 mol% under aerobic, and phosphine- and base-free conditions in water is described (Scheme 71).81 Good to high yields were obtained with arenediazonium tetrafluoroborates and potassium aryltrifluoroborates containing both electron-donating and electron-withdrawing substituents. However, a more severe steric congestion leads to trace amounts of the desired product. SEM analysis, at higher magnification, displayed aggregated nanoparticles and TEM measurements indicated that the catalyst system before use contained spheroidal palladium nanoparticles, whose average size was 3.4 ± 1.4 nm. The catalyst system could be reused several times without significant loss of activity. The material recovered after eight runs showed nanoparticles of about 3.6 ± 1.7 nm in diameter.
Hybrid organic–inorganic supports
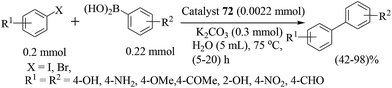
A highly active heterogeneous catalyst was prepared by Xiong et al. from coated mesoporous materials which contain a layer of readily available PEG (Scheme 74).84 Suzuki–Miyaura coupling reactions in water were performed with this catalyst at 50 °C using K3PO4 as the base (Scheme 75). The presence of electron-donating or -withdrawing groups on aryl halides or aryl boronic acids as well as steric hindrance did not have any considerable effect on the product yield under these reaction conditions. In most cases, the reaction with 0.1 mol% catalyst could afford the products in fairly good yields; the catalyst loading could even be reduced to 0.01 or 0.001 mol%. The coupling reaction was found to be effective with phenyl bromide and with the very unreactive phenyl chloride. The catalyst was highly stable and remained catalytically active after exposure to air for up to 6 weeks. An aqueous suspension of the catalyst could be reused several times by simple extraction. No leaching of the catalyst to the organic layer was observed.
The palladium-in-brush catalyst could be recovered by simple filtration. Ten consecutive preparations of 4-methoxybiphenyl showed no significant reduction in yield which was again supported by ICP-MS analyses showing low leaching of less than 0.5% of the initial palladium loading).
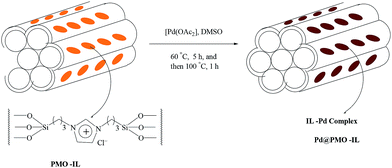
The parent ordered mesoporous organosilica with ionic liquid framework (PMO-IL) was synthesized by hydrolysis and co-condensation of 1,3-bis-(3-trimethoxysilylpropyl)imidazolium chloride and tetramethoxysilane in the presence of pluronic P123 as a template under acidic conditions. This nanostructured PMO-IL material was then reacted with a sub-stoichiometric amount of Pd(OAc)2 to afford the corresponding palladium-containing periodic mesoporous organosilica (Pd@PMO-IL) catalyst (Fig. 10) (Scheme 78).86 Pd@PMO-IL was established as an efficient and reusable catalyst for the Suzuki–Miyaura coupling reaction of various types of aryl iodides, bromides, and even deactivated chlorides in water (Scheme 79). It was also found that, although the PMO-IL nanostructure acted as a reservoir for soluble Pd species, it could also operate as a nanoscaffold to recapture Pd nanoparticles into the mesochannels thus preventing extensive agglomeration of Pd. Among the various bases screened, K2CO3 provided the highest cross-coupling yield. Highly deactivated heteroaromatic substrates led to the corresponding bis(aryl) products in excellent yields. The catalyst was also capable of activating chlorobenzene to produce the desired products in significant yields. Moreover, various functional groups, such as cyano, acyl, CHO, methyl and methoxy, were tolerated and the corresponding coupled products were obtained in good to excellent isolated yields. Surprisingly, hot filtration tests and selective catalyst poisons showed the presence of soluble Pd species during the reaction process but atomic spectroscopy and catalyst recovery studies illustrated no significant decrease in activity and metal content of recovered Pd@PMO-IL.
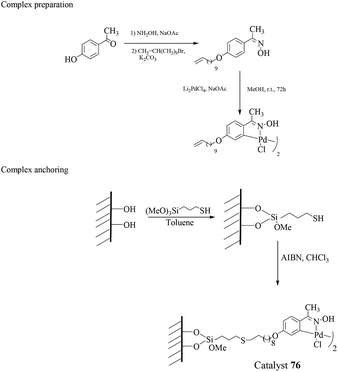 | ||
| Scheme 80 Anchoring procedure of the oxime carbapalladacycle onto the mercaptopropyl-modified high surface silica. | ||
The use of an organic solvent such as 1,4-dioxane was detrimental for the successful reaction. Moreover, the same oxime palladacycle anchored onto polymeric supports, i.e. polystyrene and polyethylene glycol bis(methacrylate), gave low conversions for the coupling products. Recycling of the Pd/SiO2 catalyst 76 was evaluated using 4-chloroacetophenone and phenylboronic acid as the substrates and indicated that eight consecutive reactions could be run without any decrease in activity. Leaching studies using the “three-phase test” were conclusive for a truly heterogeneous process with no detectable palladium species being released into the medium.
In a subsequent study, Corma and co-workers reported a related oxime palladacycle anchored onto a periodic mesoporous organosilica (PMO) (Scheme 82).89 Although the results reported by Corma and co-workers were relevant for bromoarenes, the Pd/PMO catalyst was essentially unreactive for aryl chlorides. Moreover, the recycling of Pd/PMO was limited by a significant decrease in catalytic activity upon reuse. As a consequence, PMO did not convey any benefits over the only amorphous silica.
Cai et al. also reported Suzuki–Miyaura cross-coupling reactions using perfluoro-tagged palladium nanoparticles on a fluorous silica gel (FSG) as the catalyst (Scheme 85), K2CO3 as the base and TBAB as an additive in H2O, affording the corresponding biphenyls in moderate to high yields (Scheme 86).92 It was obvious from the TEM image that the spherical palladium nanoparticles were successfully dispersed in the silica matrix with an average size of 2–3 nm. The catalyst could be recovered by simple filtration and reused several times with a slight decrease in activity.
 | ||
| Scheme 85 Preparation of fluorous nanoparticle stabilizer and the fluorous silica gel-supported Pd catalyst. | ||
Aryl bromides bearing either electron-donating or electron-withdrawing substituents in the ortho- and para-positions, afforded the corresponding biphenyls in good to excellent yields. Aryl trifluoromethanesulfonate and aryl perfluorooctanesulfonate were more active than bromobenzene in terms of yield as well as the time. However, chlorobenzene was not active in the reaction and only a moderate yield was obtained even when the catalyst was increased to up to 1 mol%. When activated aryl chloride was used, a relatively higher yield was obtained though the yield was still unsatisfactory. Recycling studies showed that the supported catalyst could be reused several times with a slight decrease in activity, and Pd leaching was less than 10 ppm.
A Pd(II) organometal catalyst with a three-dimensional (3D) cage-like Ia3d cubic mesoporous structure and a high surface area was prepared (Scheme 87).93 In comparison with the corresponding catalyst with a two-dimensional (2D) P6mm hexagonal mesoporous structure, the as-prepared catalyst exhibited higher activities in water-medium Suzuki–Miyaura coupling reactions owing to the diminished diffusion limit. It showed comparable efficiencies with the Pd(PPh3)2Cl2 homogeneous catalyst and could be easily recycled and reused for five times without significant loss of activity (Scheme 88).
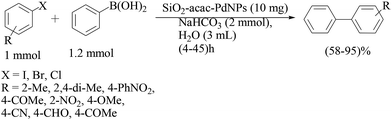 | ||
| Scheme 90 Suzuki–Miyaura coupling reaction using Pd nanoparticles on an acetyl acetone modified silica gel. | ||
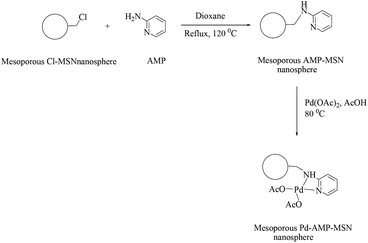
Under optimized conditions, the reaction of 4-bromoacetophenone and phenylbronic acid using 10 mg (0.0004 mmol, 0.04 mol% Pd) of the catalyst and 2 equiv. of the base gave the best result. Water was the most efficient solvent and NaHCO3 was chosen as the best base for this reaction. The catalyst could be used for cross-coupling reactions of aryl iodides, bromides and even less reactive aryl chlorides under the reflux temperature, which were transformed to the corresponding coupled products in good to excellent yields in short reaction times. However, aryl chlorides reacted more slowly in comparison to the iodide and bromide derivatives. The most hindered aryl halides usually produced the corresponding biaryls with longer reaction times and lower yields. The catalyst was recycled four times with essentially no loss of activity, even after recycling it six times, good product yields could still be obtained.
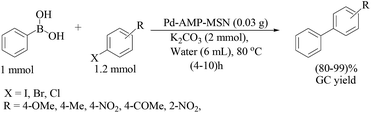 | ||
| Scheme 92 Suzuki-Miyaura cross-coupling reactions of phenyl bromide with ArB(OH)2 using the Pd–AMP-MSN catalyst. | ||
The catalytic efficiency was not significantly affected by the substituents on the aromatic ring of the halides. The Suzuki–Miyaura cross-coupling reaction of sterically hindered aryl halides could also proceed smoothly affording the desired coupled products in good yields. At the expense of time with up to 10 h, brominated heterocyclic derivatives afforded the products in good yields when treated with phenylboronic acid under the optimized conditions. The less reactive and less expensive phenyl chloride also showed moderate reactivity (yield 82–83%).
 | ||
| Scheme 97 Suzuki–Miyaura reactions of aromatic aryl halides and phenylboronic acid catalyzed by Pd–PVP/KIT-5. | ||
Following their previous work, a Pd nanoparticle–poly(2-hydroxyethyl methacrylate)/KIT-6 (Pd–PHEMA/KIT-6) composite was fabricated through an in situ polymerization method and was evaluated as a novel heterogeneous catalyst in Suzuki–Miyaura cross coupling reactions of aryl chlorides, bromides and iodides and phenylboronic acid under aerobic conditions in water by the same group of workers (Scheme 98).99 The reactions were usually carried out at 40 °C for 1 h. Sometimes a higher temperature of 95 °C as well as longer reaction times were required for the reaction depending on the nature of the reactants. The Pd content of the catalyst estimated by ICP-AES was 0.956 mmol g−1. The typical TEM micrographs showed a cubic Ia3d pore array structure for the Pd–PHEMA/KIT-6 nanocomposite. This heterogeneous catalyst could be reused at least nine times without any decrease in activity.
Conclusions
The Suzuki–Miyaura cross-coupling reaction is a method for carbon–carbon bond formation, which is a highly useful and versatile method needed for the development of modern drug discovery and the synthesis of many natural products, polymers and other organic compounds of various importance. Tremendous effort has been put forward by chemists throughout the world for improving the Suzuki–Miyaura coupling reaction, keeping in mind the current need for greener reactions. The development of sustainable chemistry is a crucial area of research for the future advancement of our society. To cope with the growing demand for eco-friendly procedures, chemists have improved heterogeneous catalytic systems and moved to safer reaction media such as water. Although a considerable progress has been achieved for the Suzuki–Miyaura coupling reaction using palladium under heterogeneous conditions, the reaction pathway under heterogeneous conditions, the minimization of leaching of palladium after a few cycles, and the exploration of other eco-friendly metal catalysts for this reaction remain still to be investigated. Nevertheless, there are still many areas to explore in the area of eco-compatible chemical synthesis, and no doubt supported organometallic chemistry will lead the way along the path of progress.Acknowledgements
SP thanks UGC for awarding DS Kothari a Post Doctoral Fellowship (no. F.4-2/2006 (BSR)/CH/13-14/0075). MMI acknowledges UGC, New Delhi, for awarding Maulana Azad a National Fellowship (F1-17.1/2013-14/MANF-2013-14-MUS-WES-24492/(SAIII). SMI acknowledges the Department of Science and Technology (DST) and Council of Scientific and Industrial Research (CSIR) New Delhi, India, for funding. We also acknowledge DST & UGC, Govt of India, for providing support to the Department of Chemistry, University of Kalyani, under the PURSE, FIST and SAP program.Notes and references
- For selected reviews, see: (a) V. Farina, Adv. Synth. Catal., 2004, 346, 1553 CrossRef CAS PubMed; (b) H.-U. Blaser, A. Indolese, F. Naud, U. Nettekoven and A. Schnyder, Adv. Synth. Catal., 2004, 346, 1583 CrossRef CAS PubMed; (c) A. Zapf and M. Beller, Chem. Commun., 2005, 431 RSC; (d) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., 2005, 117, 4516 (Angew. Chem., Int. Ed., 2005, 44, 4442) CrossRef PubMed; (e) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS PubMed; (f) J.-L. Malleron, J.-C. Fiaud and J.-Y. Legros, Handbook of Palladium Catalyzed Organic Reactions: Synthetic Aspects and Catalytic Cycles, Academic Press, San Diego, 1997 Search PubMed; (g) J. Tsuji, Palladium Reagents and Catalysts: Innovations in Organic Synthesis, Wiley, Chichester, 1995 Search PubMed; (h) S. Budagumpi, R. A. Haque and A. W. Salman, Coord. Chem. Rev., 2012, 256, 1787 CrossRef CAS PubMed; (i) E. A. B. Kantchev, C. J. O’Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed; (j) R. Stürmer, Angew. Chem., Int. Ed., 1999, 33, 3307 CrossRef.
- J.-C. Hierso, M. Beauperin and P. Meunier, Eur. J. Inorg. Chem., 2007, 3760 Search PubMed.
- (a) G. Bringmann, R. Gotz, P. A. Keller, R. Walter, M. R. Boyd, F. Lang, A. Garcia, J. J. Walsh, I. Tellitu, K. V. Bhaskar and T. R. Kelly, J. Org. Chem., 1998, 63, 1090 CrossRef CAS; (b) M. Vilaro, G. Arsequell, G. Valencia, A. Ballesteros and J. Barluenga, Org. Lett., 2008, 10, 3243 CrossRef CAS PubMed; (c) J. K. Liu, Chem. Rev., 2006, 106, 2209 CrossRef CAS PubMed.
- (a) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef CAS PubMed; (b) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (c) N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS PubMed; (d) A. Kumar, G. K. Rao, S. Kumar and A. K. Singh, Dalton Trans., 2013, 5200 RSC; (e) F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 15, 2419 Search PubMed; (f) D. A. Alonso and C. Nájera, Chem. Soc. Rev., 2010, 39, 2891 RSC; (g) B. Karimi and A. Zamani, Org. Biomol. Chem., 2012, 10, 4531 RSC.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC; (c) G. A. Molander and B. Canturk, Org. Lett., 2008, 10, 2135 CrossRef CAS PubMed.
- (a) N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437 CrossRef; (b) N. Miyaura and A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 866 RSC.
- For the first examples of alkyl–alkyl Suzuki-Miyaura cross-coupling reactions, see: (a) T. Ishiyama, S. Abe, N. Miyaura and A. Suzuki, Chem. Lett., 1992, 691 CrossRef CAS; For more recent developments in Suzuki-Miyaura coupling reactions of alkyl halides, see: (b) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 1340 CrossRef CAS PubMed; and references therein; for a review of catalysts for cross-coupling reactions with non-activated alkyl halides, see: (c) A. C. Frisch and M. Beller, Angew. Chem., 2005, 117, 680 (Angew. Chem., Int. Ed., 2005, 44, 674) CrossRef PubMed.
- (a) D. W. Old, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 9722 CrossRef CAS; (b) A. F. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020 CrossRef CAS; (c) W. Dai, Y. Li, Y. Zhang, C. Yue and J. Wu, Chem.–Eur. J., 2008, 14, 5538 CrossRef CAS PubMed; (d) A. M. Sajith and A. Muralidharan, Tetrahedron Lett., 2012, 53, 1036 CrossRef CAS PubMed; (e) G. A. Molander, F. Beaumard and T. K. Niethamer, J. Org. Chem., 2011, 76, 8126 CrossRef CAS PubMed.
- N. Miyaura, K. Yamada, H. Suginome and A. Suzuki, J. Am. Chem. Soc., 1985, 107, 972 CrossRef CAS.
- A. L. Casalnuovo and J. C. Calabrese, J. Am. Chem. Soc., 1990, 112, 4324 CrossRef CAS.
- (a) M. Carril, R. SanMartin and E. Dominguez, Chem. Soc. Rev., 2008, 37, 639 RSC; (b) K. H. Shaughnessy, Eur. J. Org. Chem., 2006, 1827 CrossRef CAS PubMed; (c) L. Bai and J. X. Wang, Curr. Org. Chem., 2005, 9, 535 CrossRef CAS; (d) K. H. Shaughnessy and R. B. DeVasher, Curr. Org. Chem., 2005, 9, 585 CrossRef CAS; (e) R. Franzen, Y. J. Can, J. Xu and C. Chim, Chem. Rev., 2005, 83, 266 CAS; (f) J. P. Genet and M. Savignac, J. Organomet. Chem., 1999, 576, 305 CrossRef.
- (a) I. D. Kostas, A. G. Coutsolelos, G. Charalambidis and A. Skondra, Tetrahedron Lett., 2007, 48, 6688 CrossRef CAS PubMed; (b) S. Gülcemal, I. Kani, F. Yilmaz and B. Cetinkaya, Tetrahedron, 2010, 66, 5602 CrossRef PubMed; (c) T. Tu, X. K. Feng and Z. X. Wang, Dalton Trans., 2010, 10598 RSC; (d) S. Q. Bai and T. S. A. Hor, Chem. Commun., 2008, 3172 RSC; (e) M. Beller, J. G. E. Krauter and A. Zapf, Angew. Chem., Int. Ed., 1997, 36, 772 CrossRef CAS PubMed; (f) K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6173 CrossRef CAS PubMed; (g) N. Liu, C. Liu and Z. L. Jin, Green Chem., 2012, 14, 592 RSC.
- (a) J. Zhi, D. P. Song and Z. W. Li, Chem. Commun., 2011, 47, 10707 RSC; (b) B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem., 2011, 76, 4379 CrossRef CAS PubMed; (c) A. Krasovskiy, I. Thomé, J. Graff, V. Krasovskaya, P. Konopelski, C. Duplais and B. H. Lipshutz, Tetrahedron Lett., 2011, 52, 2203 CrossRef CAS PubMed; (d) M. J. Jin and D. H. Lee, Angew. Chem., Int. Ed., 2010, 49, 1119 CrossRef CAS PubMed; (e) D. Zhang, C. S. Zhou and R. H. Wang, Catal. Commun., 2012, 22, 83 CrossRef CAS PubMed.
- (a) M. Mondal and U. Bora, Green Chem., 2012, 14, 1873 RSC; (b) J. Qiu, L. Wang, M. Liu, Q. Shen and J. Tang, Tetrahedron Lett., 2011, 52, 6489 CrossRef CAS PubMed.
- (a) A. L. De souza, F. L. C. da silva, B. L. Oliveira and O. A. C. Antunes, Tetrahedron Lett., 2008, 49, 3895 CrossRef CAS PubMed; (b) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed; (c) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed; (d) M. E. Hanhan, R. Martínez-Máñez and J. V. Ros-Lis, Tetrahedron Lett., 2012, 53, 2388 CrossRef CAS PubMed.
- D. G. Blackmond, A. Armstrong, V. Coombe and A. Wells, Angew. Chem., 2007, 119, 3872 (Angew. Chem., Int. Ed., 2007, 46, 3798) CrossRef PubMed.
- (a) J. Tsuji, Palladium Reagents and Catalyst: Innovations in Organic Synthesis, John Wiley & Sons Ltd, Chichester, England, 1996 Search PubMed; (b) T. Hayashi, J. Organomet. Chem., 2002, 653, 41 CrossRef CAS; (c) I. Moreno, R. San Martin, M. T. Herrero and E. Dominguez, Curr. Top. Catal., 2009, 8, 91 CAS; (d) J. Dupont and F. R. Flores, Handbook of Green Chemistry, 2009, Wiley-VCH, Weinheim, Germany, p. 319 Search PubMed.
- (a) E. B. Mubofu, J. H. Clark and D. Macquarrie, Green Chem., 2001, 3, 23 RSC; (b) D. Rosario-Amorin, X. Wang, M. Gaboyard, R. Clérac, S. Nlate and K. Heuzé, Chem.–Eur. J., 2009, 15, 12636 CrossRef CAS PubMed.
- D. J. Cole-Hamilton, Science, 2003, 299, 1702 CrossRef CAS PubMed.
- (a) N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217 CrossRef CAS PubMed; (b) T. J. Dickerson, N. N. Reed and K. D. Janda, Chem. Rev., 2002, 102, 3325 CrossRef CAS PubMed; (c) Q.-H. Fan, Y.-M. Li and A. S. C. Chan, Chem. Rev., 2002, 102, 3385 CrossRef CAS PubMed; (d) A. Molnár, Chem. Rev., 2011, 111, 2251 CrossRef PubMed; (e) I. T. Horváth and J. Rábai, Science, 1994, 266, 72 Search PubMed.
- (a) M. M Dell’Anna, M. Mali, P. Mastrorilli, A. Rizzuti, C. Ponzoni and C. Leonelli, J. Mol. Catal. A: Chem., 2013, 366, 186 CrossRef PubMed; (b) K. Bester, A. Bukowska and W. Bukowski, Appl. Catal., A, 2012, 443, 181 CrossRef PubMed; (c) S. E. S. Leonhardt, A. Stolle and B. Ondruschka, Appl. Catal., A, 2010, 379, 30 CrossRef CAS PubMed; (d) V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599 CrossRef CAS PubMed; (e) Y. S. Chun, J. Y. Shin, C. E. Song and S. G. Lee, Chem. Commun., 2008, 942 RSC; (f) C. Ramarao, S. V. Ley, S. C. Smith, I. M. Shirley and N. DeAlmeida, Chem. Commun., 2002, 1132 RSC; (g) A. Hassine, S. Sebti and A. Solhy, Appl. Catal., A, 2013, 450, 13 CrossRef CAS PubMed.
- M. Sudip, R. Gadi, J. Ashutosh, B. Mubeen and S. Yoel, Adv. Synth. Catal., 2002, 344, 348 CrossRef.
- M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33 CrossRef CAS PubMed.
- V. V. Bykov and N. A. Bumagin, Russ. Chem. Bull., 1997, 46, 1344 CrossRef CAS.
- H. Sakurai, T. Tsukuda and T. Hirao, J. Org. Chem., 2002, 67, 2721 CrossRef CAS PubMed.
- A. Arcadi, G. Cerichelli, M. Chiarini, M. Correa and D. Zorzan, Eur. J. Org. Chem., 2003, 4080 CrossRef CAS PubMed.
- G. Lu, R. Franzen, Q. Zhang and Y. Xu, Tetrahedron Lett., 2005, 46, 4255 CrossRef CAS PubMed.
- (a) M. Lysen and K. Kohler, Synlett, 2005, 1671 CrossRef CAS PubMed; (b) M. Lysen and K. Kohler, Synthesis, 2006, 692 CrossRef CAS.
- J. S. Freundlich and H. E. Landis, Tetrahedron Lett., 2006, 47, 4275 CrossRef CAS PubMed.
- R. K. Arvela and N. E. Leadbeater, Org. Lett., 2005, 7, 2101 CrossRef CAS PubMed.
- L. Bai, Chin. Chem. Lett., 2009, 20, 158 CrossRef CAS PubMed.
- J. A. Sullivan, K. A. Flanagan and H. Hain, Catal. Today, 2009, 145, 108 CrossRef CAS PubMed.
- Y. Li, X. Fan, J. Qi, J. Ji, S. Wang, G. Zhang and F. Zhang, Nano. Res., 2010, 3, 429 CrossRef CAS.
- K. Shimizu, R. Maruyama, S. Komai, T. Kodoma and Y. Kitayama, J. Catal., 2004, 227, 202 CrossRef CAS PubMed.
- M. L. Kantam, S. Roy, M. Roy, B. Sreedhar and B. M. Choudary, Adv. Synth. Catal., 2005, 347, 2002 CrossRef CAS PubMed.
- G. Durgun, O. Aksin and L. Artok, J. Mol. Catal. A: Chem., 2007, 278, 189 CrossRef CAS PubMed.
- A. Monopoli, A. Nacci, V. Calò, F. Ciminale, P. Cotugno, A. Mangone, L. C. Giannossa, P. Azzone and N. Cioffi, Molecules, 2010, 15, 4511 CrossRef CAS PubMed.
- N. Jamwal, M. Gupta and S. Paul, Green Chem., 2008, 10, 999 RSC.
- A. Indra, C. S. Gopinath, S. Bhaduri and G. K. Lahiri, Catal. Sci. Technol., 2013, 3, 1625 CAS.
- D. D. Das and A. Sayari, J. Catal., 2007, 246, 60 CrossRef CAS PubMed.
- M. Mora, C. Jinez-Sanchidrin and J. R. Ruiz, J. Mol. Catal. A: Chem., 2008, 285, 79 CrossRef CAS PubMed.
- C. Schmçger, T. Szuppa, A. Tied, F. Schneider, A. Stolle and B. Ondruschka, ChemSusChem, 2008, 1, 339 CrossRef PubMed.
- H. Ayoub, S. Saïd, A. Solhy, Z. Mohamed, L. Christophe, N. H. Mohamed and F. Aziz, Appl. Catal., A, 2013, 450, 13 CrossRef PubMed.
- S. E. Lyubimov, A. A. Vasil’ev, A. A. Korlyukov, M. M. Ilyin, S. A. Pisarev, V. V. Matveev, A. E. Chalykh, S. G. Zlotin and V. A. Davankov, React. Funct. Polym., 2009, 69, 755 CrossRef CAS PubMed.
- A. Ohtaka, T. Teratani, R. Fujii, K. Ikeshita, O. Shimomura and R. Nomura, Chem. Commun., 2009, 7188 RSC.
- A. Ohtaka, T. Teratani, R. Fujii, K. Ikeshita, T. Kawashima, K. Tatsumi, O. Shimomura and R. Nomura, J. Org. Chem., 2011, 76, 4052 CrossRef CAS PubMed.
- A. Ohtaka, Y. Kono, T. Teratani, S. Fujii, S. Matsuzawa, Y. Nakamura and R. Nomura, Catal. Lett., 2011, 141, 1097 CrossRef CAS.
- L. Bai and J.-X. Wang, Adv. Synth. Catal., 2008, 350, 315 CrossRef CAS PubMed.
- Y. Uozumi, H. Danjo and T. Hayashi, J. Org. Chem., 1999, 64, 3384 CrossRef CAS.
- Y. Uozumi and Y. Nakai, Org. Lett., 2002, 4, 2997 CrossRef CAS PubMed.
- Y. Uozumi, Y. Matsuura, T. Arakawa and Y. M. A. Yamada, Angew. Chem., 2009, 121, 2746 (Angew. Chem., Int. Ed., 2009, 48, 2708) CrossRef PubMed.
- (a) Y. M. A. Yamada, K. Takeda, H. Takahashi and S. Ikegami, Org. Lett., 2002, 4, 3371 CrossRef CAS PubMed; (b) Y. M. A. Yamada, K. Takeda, H. Takahashi and S. Ikegami, J. Org. Chem., 2003, 68, 7733 CrossRef CAS PubMed.
- Y. M. A. Yamada, Y. Maeda and Y. Uozumi, Org. Lett., 2006, 8, 4259 CrossRef CAS PubMed.
- W. Solodenko, U. Schon, J. Messinger, A. Glinschert and A. Kirschning, Synlett, 2004, 1699 CAS.
- (a) K. M. Dawood and A. Kirschning, Tetrahedron, 2005, 61, 12121 CrossRef CAS PubMed; (b) W. Solodenko, C. Brochwitz, R. Wartchow, M. A. Hashem, K. M. Dawood, M. Vaultier and A. Kirschning, Mol. Diversity, 2005, 9, 333 CrossRef CAS PubMed.
- W. Solodenko, K. Mennecke, C. Vogt, S. Gruhl and A. Kirschning, Synthesis, 2006, 1873 CAS.
- (a) D. A. Alonso, C. Najera and M. C. Pacheco, Org. Lett., 2000, 2, 1823 CrossRef CAS PubMed; (b) E. Alacid, D. A. Alonso, L. Botella, C. Najera and M. C. Pacheco, Chem. Rec., 2006, 6, 117 CrossRef CAS PubMed; (c) E. Alacid and C. Najera, Org. Lett., 2008, 10, 5011 CrossRef CAS PubMed; (d) E. Alacid and C. Najera, J. Org. Chem., 2009, 74, 2321 CrossRef CAS PubMed.
- E. Alacid and C. Najera, J. Organomet. Chem., 2009, 694, 1658 CrossRef CAS PubMed.
- (a) C. Najera, J. Gil-Molt, S. Karlstrçm and L. R. Falvello, Org. Lett., 2003, 5, 1451 CrossRef CAS PubMed; (b) C. Najera, J. GilMolt and S. Karlstrom, Adv. Synth. Catal., 2004, 346, 1798 CrossRef CAS PubMed; (c) J. Gil-Molt and C. Najera, Eur. J. Org. Chem., 2005, 4073 CrossRef PubMed.
- J. Gil-Molt, S. Karlstrom and C. Najera, Tetrahedron, 2005, 61, 12168 CrossRef PubMed.
- F. Wen, W. Zhang, G. Wei, Y. Wang, J. Zhang, M. Zhang and L. Shi, Chem. Mater., 2008, 20, 2144 CrossRef CAS.
- T. Suzuka, K. Kimura and T. Nagamine, Polymers, 2011, 3, 621 CrossRef CAS PubMed.
- T. Suzuka, T. Nagamine, K. Ogihara and M. Higa, Catal. Lett., 2010, 139, 85 CrossRef CAS.
- S. M. Islam, P. Mondal, A. S. Roy, S. Mondal and D. Hossain, Tetrahedron Lett., 2010, 51, 2067 CrossRef CAS PubMed.
- F. Siga, H. Temel, M. Aydemir, Y. S. Ocaka, S. Pasa and A. Baysal, Appl. Catal., A, 2012, 449, 172 CrossRef CAS PubMed.
- M. Bakherad, A. Keivanloo, B. Bahramian and S. Jajarmi, J. Organomet. Chem., 2013, 724, 206 CrossRef CAS PubMed.
- J.-W. Kim, J.-H. Kim, D.-H. Lee and Y.-S. Lee, Tetrahedron Lett., 2006, 47, 4745 CrossRef CAS PubMed.
- M. Bakherad, A. Keivanloo, A. H. Amin and S. Jajarmi, C. R. Chim., 2012, 15, 945 CrossRef CAS PubMed.
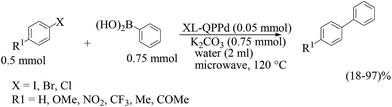
- Y. Yu, T. Hu, X. Chen, K. Xu, J. Zhang and J. Huang, Chem. Commun., 2011, 47, 3592 RSC.
- A. N. Kashin and I. P. Beletskaya, Russ. J. Org. Chem., 2011, 47, 475 CrossRef CAS.
- J. K. Cho, R. Najman, T. W. Dean, O. Ichihara, C. Muller and M. Bradley, J. Am. Chem. Soc., 2006, 128, 6276 CrossRef CAS PubMed.
- L. Wang and P.-H. Li, Chin. J. Chem., 2006, 24, 770 CrossRef CAS PubMed.
- K. H. Liew, P. L. Loh, J. C. Juan, M. A. Yarmo and R. M. Yusop, The Scientific World, 2014, 796196, DOI:10.1155/2014/796196.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., 2007, 119, 7389 (Angew. Chem., Int. Ed., 2007, 46, 7251) CrossRef PubMed.
- M. L. Kantam, M. Roy, S. Roy, B. Sreedhar, S. S. Madhavendra, B. M. Choudary and R. L. De, Tetrahedron, 2007, 63, 8002 CrossRef CAS PubMed.
- K. S. Sivudu, N. M. Reddy, M. N. Prasad, K. M. Raju, Y. M. Mohan, J. S. Yadav, G. Sabitha and D. Shailaja, J. Mol. Catal. A: Chem., 2008, 295, 10 CrossRef CAS PubMed.
- M. C. Hong, M. C. Choi, Y. W. Chang, Y. Lee, J. Kim and H. Rhee, Adv. Synth. Catal., 2012, 354, 1257 CrossRef CAS PubMed.
- S. S. Yi, D. H. Lee, E. Sin and Y. S. Lee, Tetrahedron Lett., 2007, 48, 6771 CrossRef CAS PubMed.
- E. Sin, S.-S. Yi and Y.-S. Lee, J. Mol. Catal. A: Chem., 2010, 315, 99 CrossRef CAS PubMed.
- J. Lasri, T. C. O. Mac Leod and A. J. L. Pombeiro, Appl. Catal., A, 2011, 397, 94 CrossRef CAS PubMed.
- S. Cacchi, E. Caponetti, M. A. Casadei, A. D. Giulio, G. Fabrizi, G. Forte, A. Goggiamani, S. Moreno, P. Paolicelli, F. Petrucci, A. Prastaro and M. L. Saladino, Green Chem., 2012, 14, 317 RSC.
- H.-C. Ma, W. Cao, Z.-K. Bao and Z.-Q. Lei, Catal. Sci. Technol., 2012, 2, 2291 CAS.
- C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045 CrossRef CAS PubMed.
- Q. Yang, S. Ma, J. Li, F. Xiao and H. Xiong, Chem. Commun., 2006, 2495 RSC.
- J.-F. Wei, J. Jiao, J.-J. Feng, J. Lv, X.-R. Zhang, X.-Y. Shi and Z.-G. Chen, J. Org. Chem., 2009, 74, 6283 CrossRef CAS PubMed.
- B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Chem.–Eur. J., 2010, 16, 8047 CrossRef CAS PubMed.
- (a) L. Botella and C. Najera, Angew. Chem., 2002, 114, 187 (Angew. Chem., Int. Ed., 2002, 41, 179) CrossRef; (b) D. A. Alonso, C. Najera and M. C. Pacheco, J. Org. Chem., 2002, 67, 5588 CrossRef CAS PubMed; (c) L. Botella and C. Najera, J. Organomet. Chem., 2002, 663, 46 CrossRef CAS; (d) D. A. Alonso, L. Botella, C. Najera and M. C. Pacheco, Synthesis, 2004, 1713 CAS.
- (a) C. Baleizao, A. Corma, H. Garcia and A. Leyva, Chem. Commun., 2003, 606 RSC; (b) C. Baleizao, A. Corma, H. Garcia and A. Leyva, J. Org. Chem., 2004, 69, 439 CrossRef CAS PubMed.
- A. Corma, D. Das, H. Garca and A. Leyva, J. Catal., 2005, 229, 322 CrossRef CAS PubMed.
- C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045 CrossRef CAS PubMed.
- C. C. Tzschucke and W. Bannwarth, Helv. Chim. Acta, 2004, 87, 2882 CrossRef CAS PubMed.
- L. Wang and C. Cai, J. Mol. Catal. A: Chem., 2009, 306, 97 CrossRef CAS PubMed.
- Z. Fengxia and L. Hexing, Chin. J. Chem., 2012, 30, 2151 CrossRef PubMed.
- A. R. Hajipour, Z. Shirdashtzade and G. Azizi, J. Chem. Sci., 2014, 126, 85 CrossRef CAS.
- P. Mondal, S. Banerjee, A. S. Roy, T. K. Mandal and S. M. Islam, J. Mater. Chem., 2012, 22, 20434 RSC.
- M.-J. Jin, A. Taher, H.-J. Kang, M. Choi and R. Ryoo, Green Chem., 2009, 11, 309 RSC.
- A. Taher, J.-B. Kim, J.-Y. Jung, W.-S. Ahn and M.-J. Jin, Synlett, 2009, 2477 CAS.
- R. J. Kalbasi and N. Mosaddegh, J. Solid State Chem., 2011, 184, 3095 CrossRef CAS PubMed.
- R. J. Kalbasi and N. Mosaddegh, J. Inorg. Organomet. Polym., 2012, 22, 404 CrossRef CAS.
- B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |