Fabrication and enhanced simulated sunlight photocatalytic activity of metallic platinum and indium oxide codoped titania nanotubes†
Fengyan Ma*a,
Zhi Gengb,
Xia Yang*b and
Jiyan Lengc
aCollege of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, Heilongjiang, PR China. E-mail: mafengyan989@163.com; Tel: +86 452 2742575
bSchool of Environment, Northeast Normal University, Changchun 130117, PR China
cFirst Hospital of Jilin University, Jilin University, Changchun 130021, PR China. E-mail: yangx881@nenu.edu.cn; Tel: +86 431 89165610
First published on 15th May 2015
Abstract
Metallic platinum and indium oxide codoped titania nanotubes were fabricated by a multicomponent assembly combined with a solvothermal treatment. The structure, morphology, and optical properties of the prepared samples were comparatively characterized. The Pt/In2O3–TiO2 nanotubes exhibited an anatase phase with homogeneously dispersed metallic Pt nanoparticles on the surface. Under the simulated sunlight irradiation, the photocatalytic performance of the samples was tested in the degradation of the dyes rhodamine B (RB) and diethyl phthalate (DEP). Compared to In2O3–TiO2 and TiO2 nanotubes as well as Pt/In2O3–TiO2 nanoparticles, Pt/In2O3–TiO2 nanotubes with 0.8% Pt and 9.4% In2O3 doping exhibited higher photocatalytic activity, and nearly total degradation of the dyes RB (20 mg L−1) or DEP (10 mg L−1) was obtained after 50 or 45 min under simulated light irradiation. The reasons for the enhanced photocatalytic activity were revealed.
1. Introduction
Recently, one-dimensional (1D) TiO2 nanomaterials have attracted great attention because of their interesting properties and widespread applications in the photovoltaic, photocatalytic, electrochemical, and gas sensing fields.1,2 Among the various 1D TiO2 nanomaterials, TiO2 nanotubes are currently one of the most promising photocatalysts. In comparison to TiO2 nanoparticles, the open mesoporous morphology of TiO2 nanotubes has many useful features as follow:3–5 (i) channelled structure can efficiently transfer electrons along the 1D path; (ii) high surface area enables it to adsorb a large amount of chemicals; (iii) the unique structure makes better use of light through multiple reflections within its hollow space. Therefore, TiO2 nanotubes can be served not only as the photocatalysts but also as good substrates for the enhancement of photocatalytic activity by modification. Although TiO2 possessed such promising potentials, its relatively poor charge transport property and wide bandgap (∼3.2 eV) are two main limitations for its contemporary applications in catalysis and energy harvesting/storage.However, the overall quantum efficiency of pure TiO2 is relatively low. A key issue in developing new types of function TiO2 materials is how to improve their photocatalytic efficiency. Herein a metallic platinum particles and indium oxide comodified titania nanotube system (Pt/In2O3–TiO2-NT) was demonstrated. On the one hand, In2O3 is a low band gap (2.8 eV) semiconductor, the incorporation of In2O3 is expected to extend the light response of TiO2 to a higher wavelength region and thereby decreasing its band gap;6 meanwhile, TiO2 coupled with In2O3 may promote the efficient separation of the hVB+–eCB− pairs and therefore increasing the quantum yield.7 On the other hand, deposition of metallic platinum particles throughout TiO2 nanotubes is based on their surface plasmon effects, which can lead to field enhancement in the vicinity of Pt particles and thus allowing more efficient charge transfer by capturing the eCB−.8
So far three main routes have been developed to make TiO2 nanotubes, i.e. via templating, anodic oxidation, and hydrothermal synthesis methods.9,10 The extensive applications of the templating method may be limited due to the cost, insufficient characterization of template, and a concern over long-term instability of TNT products. The anodic oxidation method is time-consuming and special conditions are needed. Furthermore, this preparation process suffers from an environmental concern, as the anodization of Ti foil must be processed in highly toxic hydrofluoric acid aqueous solutions. The sizes or diameters of the TiO2 nanomaterials obtained from the template and electrochemical anodization methods are often large, which lead to the low BET surface areas and disadvantages for the photocatalytic reactions.11,12 However, hydrothermal synthesis method has been adapted to produce high yields of TNTs with vast pore and unique nanotubular structures. As for incorporation of the components within TiO2 nanotubes, ion-exchange or impregnation method is generally applied.13 The process suffers from problems such as leaching, aggregation and poor control over the loadings of the doped component. In the case of noble metal doping, the generally used photodeposition and chemical reduction methods have the disadvantages of hard control of the size of the metal and severe aggregation of the metal clusters.14–16 Therefore, the new requirements on the preparation route are put forward.
In the present work, Pt/In2O3–TiO2 nanotubes (Pt/In2O3–TiO2-NT) were fabricated by multicomponent assembly approach combined with solvothermal treatment using titanic acid nanotubes as the staring materials in the absence of any reducing agents or stabilizing ligands. Subsequently, their simulated sunlight photocatalytic activity was evaluated by the degradation of dye rhodamine B (RB) and a kind of light insensitive compound, diethyl phthalate (DEP). Furthermore, the photoelectrochemical experiment indicated that the separation efficiency of the photo-generated electron–hole pairs was largely improved, and this led to a much higher photocatalytic activity for organic pollutant degradation. The relationship between the morphology, structure, optical properties and the photocatalytic activities of Pt/In2O3–TiO2-NTs was investigated in detail.
2. Experimental
2.1. Catalyst preparation
2.2. Preparation of Pt/In2O3–TiO2-NTs/Ti electrode
The rectangle titanium (Ti) sheets (size 10 × 50 mm, thickness 140 μm, purity > 99.6%) were cleaned ultrasonically in water and alcohol for 10 min, respectively. The cleaned Ti sheets were chemically etched in a mixture of HF, HNO3, and H2O for 30 s (HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3
HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v/v/v) followed by rinsing with distilled water and drying in argon flow at room temperature. The obtained Pt/In2O3–TiO2-NTs were well dispersed in ethanol solution under vigorous stirring for 3 h at room temperature in an open beaker. The obtained suspension was used for spin-coating Ti sheet at an initial spin rate of 5000 rpm for 6 s and then 2000 rpm for 6 s. After aging at room temperature for 12 h, the sheet was washed three times with water, and then dried at 353 K overnight. For a comparison, TiO2-NTs/Ti, In2O3–TiO2-NTs/Ti, and Pt/In2O3–TiO2-NPs/Ti photoanodes were also prepared by the same process.
5; v/v/v) followed by rinsing with distilled water and drying in argon flow at room temperature. The obtained Pt/In2O3–TiO2-NTs were well dispersed in ethanol solution under vigorous stirring for 3 h at room temperature in an open beaker. The obtained suspension was used for spin-coating Ti sheet at an initial spin rate of 5000 rpm for 6 s and then 2000 rpm for 6 s. After aging at room temperature for 12 h, the sheet was washed three times with water, and then dried at 353 K overnight. For a comparison, TiO2-NTs/Ti, In2O3–TiO2-NTs/Ti, and Pt/In2O3–TiO2-NPs/Ti photoanodes were also prepared by the same process.
2.3. Catalyst characterization
In2O3 and Pt loadings were determined by a Leeman Prodigy Spec ICP-AES. X-ray diffraction patterns were obtained on a D/max-2200 VPC diffractometer using Cu Kα radiation. XPS was performed on a VG-ADES 400 instrument with Mg Kα-ADES source at a residual gas pressure of below 10−8 Pa. Raman scattering spectra were recorded on a Jobin-Yvon HR 800 instrument with an Ar+ laser source of 488 nm wavelength in a macroscopic configuration. Nitrogen porosimetry measurement was performed on a Micromeritics ASAP 2020M surface area and porosity analyzer after the samples were outgassed under vacuum at 363 K for 1 h and 423 K for 6 h. Field-emissing scanning electron microscope (FESEM) images were obtained on a XL-30 ESEM FEG field emission scanning electron microscope at 20 kV. Transmission electron microscope (TEM) and high resolution transmission electron microscope (HRTEM) were taken on a JEM-2100F at an accelerating voltage of 200 kV. UV-vis/DRS were recorded on a Cary 500 UV-vis-NIR spectrophotometer. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet Magna 560 IR spectrophotometer. Changes of total organic carbon (TOC) in DEP degradation systems were monitored by a Shimadzu TOC-500 Total Organic Carbon analysis system.2.4. Photocatalytic tests
The simulated sunlight irradiation was supplied by a self-made solar simulator equipped with a 350 W xenon lamp and an IR cut filter. The equipment can provide uniform solar irradiance with the intensity from 0.1 W cm−2 (AM1.5G artificial sunlight, ASTM E927-05 Class A standard) to 0.3 W cm−2; meanwhile, its spectrum matches well with the natural solar light with main emission from 320 nm to 680 nm. The photocatalytic reaction was carried out in a self-made quartz photoreactor with a diameter of 63 mm. The suspension containing the solid catalyst (100 mg) and an aqueous solution of RB (20 mg L−1, 100 mL) or DEP (10 mg L−1, 100 mL) was ultrasonicated for 10 min and then stirred for 60 min in the dark. The reaction temperature was maintained at 303 ± 2 K by circulation of water through an external cooling jacket. Decreases of the concentrations of RB were analyzed by a Cary 500 UV-vis-NIR spectrophotometer at λ = 553 nm. Changes of DEP concentrations were monitored by a Shimadzu LC-20A HPLC: C18 column, UV detector (λ = 227 nm), acetonitrile/water (80/20 v/v), and 0.9 mL min−1.2.5. Photoelectrochemical experiment
Photocurrent measurement was carried out using the conventional three electrode setup connected to an electrochemical station (CH Instrument 660C, Shanghai Chenhua, China). In this electrochemical system, Pt/In2O3–TiO2-NTs/Ti, In2O3–TiO2-NTs/Ti, TiO2-NTs/Ti or Pt/In2O3–TiO2-NPs/Ti (effective area is 4 cm2) was used as a working photoanode, and a Pt sheet (size 30 × 40 mm, purity 99.99%) and an Ag/AgCl (saturated KCl) electrode were used as the counter electrode and reference electrode, respectively. The electrolyte was 0.01 mol L−1 Na2SO4 aqueous solution (110 mL), and the distance between Ti sheet and Pt sheet was fixed at 30 mm. An external 125 W high-pressure mercury lamp was used as light source. The distance between Ti sheet and light source was fixed at 80 mm. The measurements were carried out at a constant potential of +1.0 V to the working photoanode.3. Results and discussion
3.1. Catalyst characterization
The crystalline phases of as-prepared composites with various Pt dopings (0 to 6.9%) were further studied (Fig. 1b). The major phase of all the tested samples was the anatase structure regardless of Pt loadings in the products. Up to 0.8 wt%, no diffractions corresponding to Pt were observed, which indicated that Pt was dispersed uniformly in the TiO2-NT. The Pt/In2O3–TiO2-NTs-(6.9, 12.0) showed peaks at 2θ values of 39.8° and 46.3°, which are the characteristic peaks corresponding to the (111) and (200) planes of the facecentered cubic crystal of metallic platinum nanoparticles.18 Fig. 1c showed the XRD patterns of the Pt/In2O3–TiO2-NTs-(0.8, 9.4) composites obtained at different calcination temperatures. It is found that all the tested samples exhibited anatase phase. Moreover, these diffraction peaks became sharper as the calcination temperature was increased.
The surface chemical composition of the as-prepared TiO2-based nanotubes was investigated by XPS technique. As shown in Fig. 2a, the determined binding energies of Ti 2p3/2 and Ti 2p1/2 were 458.1 eV and 463.8 eV, respectively, characteristic of the Ti(IV) oxidation state in the Ti–O bonds of bulk TiO2 nanotubes. After introduction of the In2O3 or In2O3 and Pt into the TiO2 nanotubes, the binding energies of Ti 2p3/2 and Ti 2p1/2 shifted to higher values. It indicates that a slight perturbation of titanium environment occurred due to In2O3 doping or In2O3 and Pt co-doping. The typical high-resolution XPS spectra of In 3d were displayed in Fig. 2b. The peaks at 444.6 eV and 452.2 eV (for Pt/In2O3–TiO2-NTs-(0, 9.4)), and 444.9 eV and 452.2 eV (for Pt/In2O3–TiO2-NTs-(6.9, 9.4)) were signed to In 3d5/2 and In 3d3/2, respectively, which were characteristic of In3+ in In2O3.6 As to the high-resolution XPS spectrum of the Pt 4f, two peaks at 70.3 and 74.0 eV in Pt/In2O3–TiO2-NTs-(6.9, 9.4) sample were signed to metallic platinum, indicating that the platinum species existing in the product was metallic Pt (Pt0) rather than Pt2+ or Pt4+ ion.19,20 Thus, we can confirm that the In2O3 or In2O3 and Pt successfully doped in TiO2-NTs.
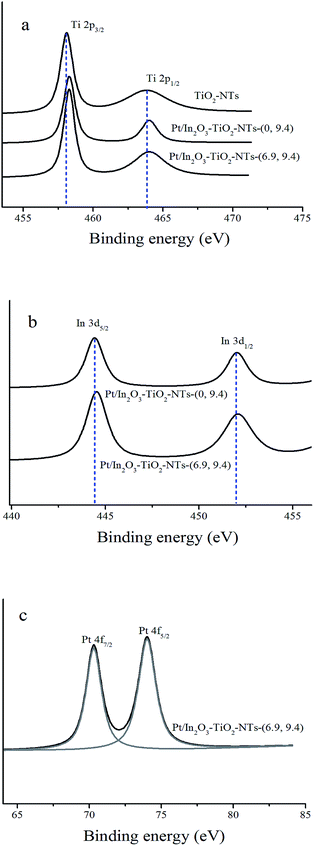 | ||
| Fig. 2 XPS spectra of the TiO2-NTs, Pt/In2O3–TiO2-NTs-(0, 9.4), and Pt/In2O3–TiO2-NTs-(6.9, 9.4) materials in the Ti 2p (a), In 3d (b), and Pt 4f (c) binding energy regions. | ||
Fig. 3 showed the Raman spectra of TiO2-based nanotubes. For comparison, TiO2 nanoparticles (TiO2-NPs) were tested under the same conditions. TiO2-NPs were characterised by well-defined peaks at 144 cm−1 (Eg), 399 cm−1 (B1g), 513 cm−1 (A1g), and 639 cm−1 (Eg) which corresponded to the anatase structure.21 As for the TiO2-NTs, in addition to anatase peaks, the 280 cm−1 Raman peak of TiO2-NTs appeared after forming the tubular structure and was associated with Ti–O–H bond resulting from proton exchange with HCl; the peak at 450 cm−1 was assigned to the Ti–O–Ti vibrations, whereas the bands at 700 cm−1 and 917 cm−1 were attributed to Ti–O–Na vibrations in the interlayer regions of the nanotube walls.22,23 As for the In2O3–TiO2-NTs-(0, 9.4) and Pt/In2O3–TiO2-NTs-(0.8, 9.4), the characteristic peak at 639 cm−1 (Eg) become intensively sharper; however, peaks at 280 cm−1 and 450 cm−1 became weaken, even peaks at 700 cm−1 and 917 cm−1 completely disappeared. These results revealed that the order degree of tubular structures of the composite reduced, but still maintained their tubular structures.
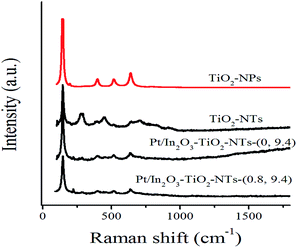 | ||
| Fig. 3 Raman spectra of as-prepared TiO2-NPs, TiO2-NTs, Pt/In2O3–TiO2-NTs-(0, 9.4), and Pt/In2O3–TiO2-NTs-(0.8, 9.4) materials. | ||
The nitrogen adsorption–desorption isotherms of the TiO2-based samples were presented in Fig. 4a. The adsorption–desorption isotherms of TiO2-based nanotubes indicated the type IV with H3 hysteresis loops, which according to BDDT classification was characteristic of a mesoporous material having pore sizes between 2 and 50 nm.24 This was confirmed by the pore size distribution (Fig. 4b). Moreover, the formation of such mesoporous materials was attributed to the aggregation of the primary nanocrystallites. In the case of Pt/In2O3–TiO2-NPs, its adsorption isotherm showed a Type IV with H2 hysteresis loop, which resulted from the synthesis methods difference. The textural parameters including BET surface areas, pore volumes, and median pore diameters derived from N2-adsorption measurements are summarized in Table 1 (ESI†). From the above results it is found that doping In2O3 or In2O3 and Pt with TiO2-based nanotubes resulted in decreases of BET surface areas (250.8–65.1 m2 g−1) and that higher In2O3 or Pt loadings led to the smaller BET surface areas. As for the Pt/In2O3–TiO2-NPs (134.8 m2 g−1), its BET surface area was slightly larger than that of the corresponding Pt/In2O3–TiO2-NTs (123.7 m2 g−1), which resulted from the synthesis methods difference. In addition, with increasing in the calcination temperature from 573 K to 773 K, the BET surface area of the Pt/In2O3–TiO2-NTs-(0.8, 9.4) gradually decreased from 237.6 m2 g−1 to 64.1 m2 g−1.
 | ||
| Fig. 5 FESEM and TEM images of TiO2-NTs (a and b), Pt/In2O3–TiO2-NTs-(0, 9.4) (c and d), and Pt/In2O3–TiO2-NTs-(0.8, 9.4) (e and f), respectively. HRTEM images of Pt/In2O3–TiO2-NTs-(0.8, 9.4) (g). | ||
The connecting mode of Pt, In2O3, and TiO2 in the Pt/In2O3–TiO2-NTs material can be inferred by the combination of ionic radius data of In3+ and Ti4+ and TEM observation. That is, the ionic radius of the doping In3+ ion (81 pm) is larger than that of the lattice Ti4+ ion (60.5 pm).26 Accordingly, it is difficult for the In3+ ions to enter into the lattice of TiO2, but it is energetically favorable for In3+ to reside in the interstitial site of TiO2 lattice.27 We therefore tentatively infer that the In2O3 homogeneously disperse throughout TiO2 framework in the Pt/In2O3–TiO2-NTs material, and both In2O3 and TiO2 construct the wall structure of the nanotubes together; simultaneously, Pt nanoparticles (average size of 5 nm) are well distributed on both the inner and outer surfaces of the nanotubes.
3.2. Photocatalytic test
Adsorption tests showed that: (i) it took 30 min to reach adsorption–desorption equilibrium; (ii) for the In2O3–TiO2-NTs materials, they showed enhanced adsorption capacity to RB molecules with increasing In2O3 doping from 0 to 12.0% (Fig. 7a); (iii) Pt/In2O3–TiO2-NTs materials displayed higher adsorption capacity with increasing Pt loading (Fig. 7b). This was due to In2O3 or Pt doping increasing the negative charge on the titania surface, which promoted the adsorption of cationic dye RB;28 and (iv) Pt/In2O3–TiO2-NTs-(0.8, 9.4) material obtained at lower calcination temperature showed somewhat higher adsorption capacity compared with the materials obtained at higher calcination temperature (Fig. 7c), attributed to larger BET surface area of the former with respect to the latter (Table 1 in ESI†).
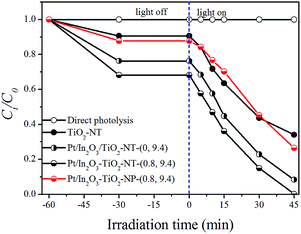
Fig. 7a showed the influence of the In2O3 loading on the photocatalytic activity of the Pt/In2O3–TiO2-NTs-(0, y). It indicates that the initial RB concentration was almost unchangeable under the solar simulating Xe lamp irradiation for 60 min. However, significant degradation of RB occurred in the presence of both the simulated sunlight irradiation and photocatalyst, it is found that the photocatalytic activity of the Pt/In2O3–TiO2-NTs-(0, y) materials to RB degradation monotonically increased with the increase of In2O3 doping from 0% to 9.4%. Further increase In2O3 doping to 12.0% caused a slight decreased photocatalytic activity.
Fig. 7b displayed the influence of the Pt loading on the photocatalytic activity of the Pt/In2O3–TiO2-NTs-(x, 9.4). It is found that the photocatalytic activity of the Pt/In2O3–TiO2-NTs materials to RB degradation monotonically increased with the increase of Pt doping from 0% to 6.9%. After 30 min simulated sunlight irradiation degradation of RB reached to 68.4% (Pt/In2O3–TiO2-NTs-(0, 9.4)), 78.2% (Pt/In2O3–TiO2-NTs-(0.4, 9.4)), 89.6% (Pt/In2O3–TiO2-NTs-(0.8, 9.4)), and 96.8% (Pt/In2O3–TiO2-NTs-(6.9, 9.4)), respectively. Therefore, Pt/In2O3–TiO2-NTs-(6.9, 9.4) showed the highest photocatalytic activity among all tested photocatalysts.
Finally, the influence of the catalyst calcination temperature on the photoactivity of Pt/In2O3–TiO2-NTs-(0.8, 9.4) was investigated in Fig. 7c. It can be observed that the simulated sunlight photocatalytic activity was enhanced as the annealing temperature increased from 573 to 673 K. Further increasing the temperature to 773 K led to the decreased activity.
Adsorption tests showed that: (i) it took 30 min to reach adsorption–desorption equilibrium between DEP molecules and the catalyst; (ii) for the TiO2-based materials with nanotube geometry, the adsorption capacity increased after the introduction of In2O3 and/or Pt regardless of their BET surface areas; (iii) compared with Pt/In2O3–TiO2-NPs, Pt/In2O3–TiO2-NTs showed stronger adsorption capacity, although both of them possessed similar BET surface area and the same Pt (0.8%) and In2O3 (9.4%) loading. The result implied the adsorption ability of the Pt/In2O3–TiO2 materials was related to their morphology characteristics. The adsorption of DEP molecules on the Pt/In2O3–TiO2 surface was due to the hydrogen bonding between the surface hydroxyl groups of TiO2 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OH) and two carbonyl oxygen atoms of DEP molecules. Therefore, the number of surface
Ti–OH) and two carbonyl oxygen atoms of DEP molecules. Therefore, the number of surface ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OH groups determined the adsorption ability of the Pt/In2O3–TiO2 materials. Compared with Pt/In2O3–TiO2-NPs, the open morphology of Pt/In2O3–TiO2-NTs can provide more surface
Ti–OH groups determined the adsorption ability of the Pt/In2O3–TiO2 materials. Compared with Pt/In2O3–TiO2-NPs, the open morphology of Pt/In2O3–TiO2-NTs can provide more surface ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OH groups to adsorb the DEP molecules, originating from the contribution of both the outer and inner surface of the materials. The conclusion is supported by FT-IR measurement (Fig. S2†), showing that the peak intensities of
Ti–OH groups to adsorb the DEP molecules, originating from the contribution of both the outer and inner surface of the materials. The conclusion is supported by FT-IR measurement (Fig. S2†), showing that the peak intensities of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OH groups (situated at 3440 cm−1 and 1637 cm−1) in the Pt/In2O3–TiO2-NTs are somewhat higher. Similar explanation was reported by Lim's work.29
Ti–OH groups (situated at 3440 cm−1 and 1637 cm−1) in the Pt/In2O3–TiO2-NTs are somewhat higher. Similar explanation was reported by Lim's work.29
Fig. 8 also indicated that the decrease of DEP concentration was negligible after the simulated sunlight irradiation for 45 min in the absence of photocatalyst. As for the TiO2-based materials, their simulated sunlight photocatalytic activity followed the order Pt/In2O3–TiO2-NTs-(0.8, 9.4) > Pt/In2O3–TiO2-NTs-(0, 9.4) > Pt/In2O3–TiO2-NPs-(0.8, 9.4) > TiO2-NTs. The above results were consistent with those obtained for RB degradation.
To clarify the final products after decomposition, we measured the total organic carbon (TOC) in the residual solution after the photodegradation of DEP. Fig. S3† shows that the changes of TOC, which indicates that many DEP molecules were destroyed to form CO2, H2O, and other small molecules. However, the TOC conversion rations are not consistent with the photodegradation ration of DEP, which indicated that a part of DEP molecules only transferred to intermediate products. After 4 h simulated sunlight irradiation, the DEP mineralization reached to 86.3% with the Pt/In2O3–-TiO2-NTs-(0.8, 9.4) photocatalyst.
3.3. Recyclability of the catalyst
It should be noted that, for a photocatalyst to be commercially viable, stability and high activity of the photocatalyst are both indispensable. Herein, Pt/In2O3–TiO2-NTs-(0.8, 9.4) was chosen to evaluate the recyclability of the catalyst. After the first catalytic run, the catalyst was removed by centrifugation and filtration, and then it was washed by hot water and ethanol for several hours, respectively. Subsequently, the catalyst was dried at 353 K for 24 h. The recovered catalyst was used for subsequent catalytic runs under the same experimental conditions. As shown in Fig. 9, no noticeable decrease of activity was observed for this photocatalyst by five times' DEP degradation reaction under simulated sunlight irradiation. The result was in accordance with the TEM and HRTEM images. As displayed in Fig. S4,† the Pt/In2O3–TiO2-NTs-(0.8, 9.4) still maintained tubular morphology and the Pt particle size became a little bigger after recycling for five times. Thus, the Pt/In2O3–TiO2-NTs-(0.8, 9.4) composite photocatalyst held an extraordinarily high stability and recyclability for photocatalytic applications.3.4. Discussion
The above photocatalytic tests indicated that (1) for the TiO2-based materials with nanotube geometry, the simulated photocatalytic activity of pure TiO2-NTs could be further increased by introduction proper In2O3 and/or Pt loading; (2) Pt/In2O3–TiO2-NTs-(0.8, 9.4) calcined at 673 K showed the highest photocatalytic activity under the different calcination temperature; (3) Pt/In2O3–TiO2-NTs-(0.8, 9.4) showed manifestly higher activity than Pt/In2O3–TiO2-NPs-(0.8, 9.4). The excellent photocatalytic activity of the Pt/In2O3–TiO2-NTs was explained by the following crucial factors.Firstly, both In2O3 and Pt co-doped TiO2 nanotubes play the beneficial roles in improving the photocatalytic activity of TiO2 by efficient separation of eCB−–hVB+ pairs. On the one hand, the conduction band (CB) bottom and the valence band (VB) top of TiO2 lie at −0.4 and 2.8 eV (NHE), while the CB bottom and VB top of In2O3 lie at −0.63 and 2.17 eV (NHE), respectively.30 Meanwhile, the band gap of In2O3 (2.8 eV) is lower than that of TiO2 (3.2 eV). As a consequence, when In2O3 couple with TiO2, the In2O3 can be excited under simulated sunlight irradiation and generates electrons in the In2O3, and then transfer to the conduction band (CB) of TiO2, which leads to efficient separation of eCB−–hVB+ pairs. On the other hand, highly dispersed Pt nanoparticles on the inner and outer surfaces of In2O3–TiO2 nanotubes can generate a Schottky barrier at the interface between Pt and TiO2, which can capture effectively eCB− and promote the overall charge separation efficiency.31 The band-gap structure of Pt/In2O3–TiO2-NTs was proposed (Scheme 1). To further confirm the above explanation, the transient photocurrent responses of TiO2-based nanotubes were measured under the UV-light irradiation. Fig. 10 depicted a comparison of photocurrent responses of different samples. It can be seen that Pt/In2O3–TiO2-NTs/Ti and TiO2-NTs/Ti electrode exhibited the highest and lowest photocurrent response, respectively. As for the Pt/In2O3–TiO2-NTs/Ti electrode, high photocurrent meant more photoinduced electrons were transferred effectively from work electrode to counter electrode via external circuit, indicating high efficiency of electron–hole pair separation and transfer by introduction In2O3 and Pt in TiO2 nanotubes.
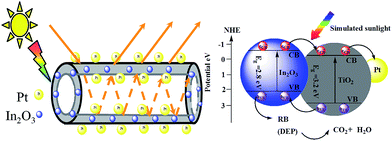 | ||
| Scheme 1 Band gap structure and the simulated sunlight photocatalytic degradation of an aqueous RB (DEP) over the Pt/In2O3–TiO2-NTs. | ||
 | ||
| Fig. 10 Photocurrent responses of TiO2-based Ti electrodes under UV light irradiation. 0.01 mol L−1 Na2SO4; the working electrode potential +1.0 V. | ||
Secondly, in comparison to nanoparticles, open mesoporous morphology of nanotubes could facilitate the transport of the substance to the active sites during the photocatalytic reaction (Scheme 1);3 meanwhile, the unique structure makes better use of light through multiple reflections within its hollow space; beyond that, the nanotubes not only provided an efficient transport channel for photogenerated electrons but also improved the dispersion of Pt and In2O3 nanoparticles throughout the nanotubes, which in turn suppressed the formation of the hVB+–eCB− recombination centers. This conclusion also can be evidenced by the photoelectrochemical experiment, showing that the photocurrent response of Pt/In2O3–TiO2-NPs/Ti electrode is lower than Pt/In2O3–TiO2-NTs/Ti electrode.
Finally, the crystallinity of the Pt/In2O3–TiO2-NTs-(0.8, 9.4) material may be increased somewhat with increase the calcinations temperature from 573 K to 673 K, the well crystallized anatase could facilitate the transfer of the photoinduced electrons from bulk to surface, which promoted efficient separation of eCB−–hVB+ pairs. When the calcination temperature increased to 773 K, the crystallinity of the sample was further enhanced. However, BET area decreases from 123.7 m2 g−1 to 64.1 m2 g−1. Meanwhile, the tubular structure began to collapse, subsequently lowered the dispersion of Pt and In2O3 throughout TiO2 nanotubes, and further increased the nanoparticles aggregation. These factors led to the decreased photocatalytic activity of Pt/In2O3–TiO2-NTs-(0.8, 9.4) with increase the catalyst calcination temperature.
4. Conclusions
Pt/In2O3–TiO2-NTs were fabricated via multicomponent assembly combined with solvothermal treatment. The prepared Pt/In2O3–TiO2-NTs system exhibited considerably high simulated sunlight photocatalytic activity towards the degradation of RB and DEP, and its catalytic activity outperformed TiO2-NTs, In2O3–TiO2-NTs, and Pt/In2O3–TiO2-NPs. This enhanced photocatalytic activity is due to more efficient separation of the hVB+–eCB− pairs, originating from the introduction of In2O3 and Pt within TiO2 framework, the nanotubular geometries, and the crystallinity of the materials. It is believed that this result is of great importance in developing new photocatalysts for pollutant degradation.Acknowledgements
This work was supported by the Natural Science Fund Council of China (21173036; 51278092; 51478095), and the Program for Young Teachers Scientific Research in Qiqihar University (2014K-M03). The work is also supported by the Program for New Century Excellent Talents in University of the Ministry of Education of China (NCET-13-0723).Notes and references
- Q. X. Deng, M. D. Wei, X. K. Ding, L. L. Jiang, B. H. Ye and K. M. Wei, Chem. Commun., 2008, 31, 3657–3659 RSC.
- X. D. Wang, Z. D. Li, J. Shi and Y. H. Yu, Chem. Rev., 2014, 114, 9346–9384 CrossRef CAS PubMed.
- T. Wang, S. Wang, W. X. Chen, W. Wang, Z. L. Xu, Y. Liu and T. Horib, J. Mater. Chem., 2009, 19, 4692–4694 RSC.
- G. L. Li, J. Y. Liu and G. B. Jiang, Chem. Commun., 2011, 47, 7443–7445 RSC.
- H. X. Guo, J. H. Chen, W. Weng, Z. S. Zheng and D. F. Wang, J. Ind. Eng. Chem., 2014, 20, 3081–3088 CrossRef CAS PubMed.
- X. Yang, Y. H. Wang, L. L. Xu, X. D. Yu and Y. H. Guo, J. Phys. Chem. C, 2008, 112, 11481–11489 CAS.
- D. Shchukin, S. Poznyak, A. Kulak and P. Pichat, J. Photochem. Photobiol., A, 2004, 162, 423–430 CrossRef CAS.
- B. K. Vijayan, N. M. Dimitrijevic, J. S. Wu and K. A. Gray, J. Phys. Chem. C, 2010, 114, 21262–21269 CAS.
- N. Liu, X. Y. Chen, J. L. Zhang and J. W. Schwank, Catal. Today, 2014, 225, 34–51 CrossRef CAS PubMed.
- N. Pugazhenthiran, S. Murugesan and S. Anandan, J. Hazard. Mater., 2013, 263, 541–549 CrossRef CAS PubMed.
- D. A. Wang, B. Yu, C. W. Wang, F. Zhou and W. M. Liu, Adv. Mater., 2009, 21, 1964–1967 CrossRef CAS PubMed.
- E. Ortel, S. Sokolov and R. Kraehnert, Microporous Mesoporous Mater., 2010, 127, 17–24 CrossRef CAS PubMed.
- M. Qamar, S. J. Kim and A. K. Ganguli, Nanotechnology, 2009, 20, 455703–455710 CrossRef CAS PubMed.
- A. C. Chen and P. Holt-Hindle, Chem. Rev., 2010, 110, 3767–3804 CrossRef CAS PubMed.
- X. J. Feng, J. D. Sloppy, T. J. LaTempa, M. Paulose, S. Komarneni, N. Z. Bao and C. A. Grimes, J. Mater. Chem., 2011, 21, 13429–13433 RSC.
- X. Y. Pan and Y. J. Xu, J. Phys. Chem. C, 2013, 117, 17996–18005 CAS.
- B. Abida, L. Chirchi, S. Baranton, T. W. Napporn, H. Kochkar, J. M. Léger and A. Ghorbel, Appl. Catal., B, 2011, 106, 609–615 CrossRef CAS PubMed.
- A. A. Ismail and D. W. Bahnemann, Green Chem., 2011, 13, 428–435 RSC.
- H. Y. Chen, Z. B. Rui and H. B. Ji, Ind. Eng. Chem. Res., 2014, 53, 7629–7636 CrossRef CAS.
- J. C. Colmenares, A. Magdziarz, M. A. Aramendia, A. Marinas, J. M. Marinas, F. J. Urbano and J. A. Navio, Catal. Commun., 2011, 16, 1–6 CrossRef CAS PubMed.
- J. G. Yu, T. T. Ma and S. W. Liu, Phys. Chem. Chem. Phys., 2011, 13, 3491–3501 RSC.
- L. Torrente-Murciano, A. A. Lapkin and D. Chadwick, J. Mater. Chem., 2010, 20, 6484–6489 RSC.
- C. Ratanatawanate, C. R. Xiong and K. J. Balkus Jr, ACS Nano, 2008, 2, 1682–1688 CrossRef CAS PubMed.
- B. J. Vijayan, N. M. Dimitrijevic, T. Rajh and K. Gray, J. Phys. Chem. C, 2010, 114, 12994–13002 CAS.
- C. X. Wang, L. W. Yin, L. Y. Zhang, N. N. Liu, N. Lun and Y. X. Qi, ACS Appl. Mater. Interfaces, 2010, 2, 3373–3377 CAS.
- L. Xu, H. W. Song, B. Dong, Y. Wang, J. S. Chen and X. Bai, Inorg. Chem., 2010, 49, 10590–10597 CrossRef CAS PubMed.
- R. Sasikala, A. R. Shirole, V. Sudarsan, Jagannath, C. Sudakar, R. Naik, R. Rao and S. R. Bharadwaj, Appl. Catal., A, 2010, 377, 47–54 CrossRef CAS PubMed.
- H. Tahiri, Y. A. Ichou and J. M. Herrmann, J. Photochem. Photobiol., A, 1998, 114, 219–226 CrossRef CAS.
- Y. W. Lim, Y. X. Tang, Y. H. Cheng and Z. Chen, Nanoscale, 2010, 2, 2751–2757 RSC.
- J. B. Mu, B. Chen, M. Y. Zhang, Z. C. Guo, P. Zhang, Z. Y. Zhang, Y. Y. Sun, C. L. Shao and Y. C. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 424–430 CAS.
- H. Q. An, J. Zhou, J. X. Li, B. L. Zhu, S. R. Wang, S. M. Zhang, S. H. Wu and W. P. Huang, Catal. Commun., 2009, 11, 175–179 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Textural parameters, TEM, HRTEM images, FT-IR spectra, and TOC of samples datas. See DOI: 10.1039/c5ra05404d |
| This journal is © The Royal Society of Chemistry 2015 |