Micro/nano-structured polyaniline/silver catalyzed borohydride reduction of 4-nitrophenol†
Abstract
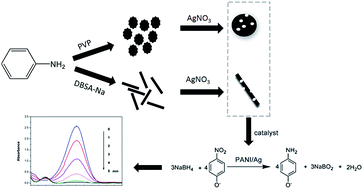
Micro/nano-structured polyaniline/silver (PANI/Ag) composites have been prepared by simply mixing nitric acid doped polyaniline with silver nitrate. A reducing agent was added to accelerate the reaction process and enhance the conversion rate of silver nitrate. As the support material of silver nanoparticles, nitric acid doped polyaniline was synthesized under turbulent flow and surfactants were adopted to control the morphology and uniformity of the particles. Various analysis techniques were adopted to confirm the composition and structure of the composites, including scanning electron microscopy (SEM), X-ray diffraction (XRD), transmission electron microscopy (TEM), Fourier transform infrared spectrometry (FTIR) and energy dispersive X-ray analysis (EDX). The PANI/Ag particles were used as a catalyst in the borohydride reduction reaction of 4-nitrophenol (4-NP), which was online monitored by UV-vis absorption spectroscopy. Results demonstrated that PANI microspheres loaded with 18.30 wt% of silver showed a comparable catalytic performance, and the apparent rate constant is 0.0256 s−1. Moreover, the stability and reusability of the catalyst were also investigated. The present work highlights the incorporation of well-dispersed silver nanoparticles with the conductive polyaniline matrix, and the accelerated electron transfer during the catalytic process owing to the high electrical conductivity of the support material.

 Please wait while we load your content...
Please wait while we load your content...