One-pot synthesis of unsymmetrical squaramides†
Abstract
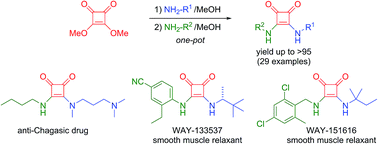
The results concerning the first one-pot synthesis of unsymmetrical squaramides are reported. This straightforward procedure allows appealing and commonly used squaramide derivatives to be obtained with very good results. The methodology developed in this work saves energy, eliminates the purification steps for intermediate products, reduces costs and leads to better yields compared to those obtained through the traditional “stop-and-go” approach. Moreover, we have proved the efficiency of our process with the synthesis of three biologically active structures, improving the results of the previously reported stepwise syntheses. Interestingly, this simple and cheap method could attract the interest of pharmaceutical and chemical companies aiming to produce these active compounds on a large scale.

 Please wait while we load your content...
Please wait while we load your content...