A comparative study of ordered mesoporous carbons with different pore structures as anode materials for lithium-ion batteries†
Abstract
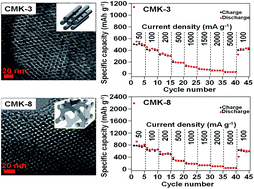
In this study, ordered mesoporous carbons (OMCs) with different pore structures, namely 2D hexagonal CMK-3 and 3D cubic CMK-8 prepared by the nanocasting method using mesoporous silicas SBA-15 and KIT-6 as hard templates, respectively, in their pure forms are used as anode materials in lithium ion batteries (LIBs) to evaluate the role of mesoporous structures in their electrochemical performances. The results demonstrate that the CMK-8 electrode exhibits a higher reversible capacity and better cycling stability and rate capability, as compared to the CMK-3 electrode, due to its unique 3D cubic mesostructure. The initial capacities of 1884 and 964 mA h g−1 are obtained for the CMK-8 and CMK-3 electrodes, respectively. The CMK-8 electrode exhibits a higher capacity value (around 37.4% higher) than the CMK-3 electrode at the 100th cycle. The enhanced electrochemical performance of CMK-8 is mainly attributable to its unique 3D channel networks, which are beneficial for efficient Li storage and volume change. Although CMK-3 is the most investigated OMCs used in LIBs, herein we demonstrate that CMK-8 is a better carbon matrix for the fabrication of the electrode materials composed of mesoporous carbons.


 Please wait while we load your content...
Please wait while we load your content...