Difunctional biogenic Au nanoparticles for colorimetric detection and removal of Hg2+†
Abstract
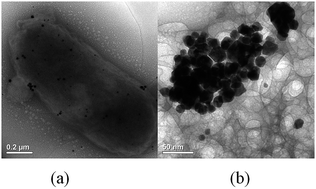
Biogenic Au nanoparticles (AuNPs) produced by Cupriavidus metallidurans SHE could act as a colorimetric sensor and scavenger of Hg2+ based on biological reduction mediated formation of an amalgam.

 Please wait while we load your content...
Please wait while we load your content...