Synthesis of chlorostannate(II) ionic liquids and their novel application in the preparation of high-quality L-lactide†
Bin Jiangabc,
Xiaowei Tantaiab,
Luhong Zhang*ab,
Li Haoa,
Yongli Suna,
Lin Dengab and
Zhiqiang Shic
aSchool of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, People's Republic of China. E-mail: zhanglvh@tju.edu.cn; Fax: +86 2227400199; Tel: +86 2227400199
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, People's Republic of China
cNational Engineering Research Centre of Distillation Technology, Tianjin University, Tianjin 300072, People's Republic of China
First published on 2nd June 2015
Abstract
Polylactic acid (PLA) is a representative biodegradable polymer, which is expected to be a promising replacement for some petroleum-based materials. Noticeably, the properties of PLA products depend strongly on the quality of the lactide monomer, a crucial precursor of PLA production. In this work, a large range of different chlorostannate(II) ionic liquids (ILs), prepared by mixing 1-butyl-3-methyl-imidazolium chloride and tin(II) chloride in various molar ratios, xSnCl2, were firstly applied for the preparation of L-lactide of high chemical and optical purity. The cation–anion interaction, the thermal stability and the acidity of imidazolium-based chlorostannate(II) ionic liquids were experimentally determined and systematically analyzed. Compared with the conventional SnCl2 catalyst, the depolymerization of oligomeric poly(L-lactic acid) catalyzed by chlorostannate(II) ionic liquids occurred in a moderate yield. Interestingly, using [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) as a catalyst, L-lactide of 99.9% optical purity was obtained, simultaneously leaving a high-Mw oligomeric residue with high isotacticity (99.1%). Furthermore, the effects of various reaction parameters were investigated in order to obtain the highest possible yield of lactide. A plausible reaction mechanism was suggested and discussed. Finally, owing to the reutilization of PLLA residue of high isotacticity, a reiterative lactide synthesis was realized. The recycled catalyst showed no notable loss of activity. By combining this chlorostannate(II)-based IL (xSnCl2 = 0.63) catalyst technology with the cyclic resynthesis process, high-quality L-lactide could be selectively produced in high yield (>80%, based on L-lactic acid replenished).
Introduction
Owing to the solid waste disposal problems caused by petroleum-based plastics, there has been a sustained research interest in the development of biodegradable polymers as replacements over the past decade.1,2 Polylactic acid (PLA) is the front runner in the emerging bioplastics market with the best availability and the most attractive cost structure.3 PLA can be produced by condensation polymerization directly from its basic building block lactic acid, which is derived from sustainable sources.4 However, it was difficult to obtain a high molecular-weight (Mw) PLA using the conventional, direct condensation reaction, owing to the existence of the dehydrated equilibrium or the resulting depolymerization reaction.5 Currently, the industrially acceptable PLA synthetic process is the ring-opening polymerization (ROP) of lactide, the six-membered dimeric cyclic esters of lactic acid.6,7 In this process, the lactic acid monomer is first polymerized to low-Mw PLA, depolymerized or dimerized to lactide, and then re-polymerized to obtain high-Mw PLA.8 This mechanism does not generate additional water, and hence a wide range of molecular weights is accessible.9 It is found that processing, crystallization and degradation behavior of PLA all depend strongly on the structure and composition of the polymer chains.10 Isotactic poly(L-lactic acid) (PLLA) has high melting point, high crystallinity, and then better mechanical properties, such as higher modulus, than poly(D,L-lactic acid) (PDLLA).3,11 Thus, PLLA is usually used for an alternative to the petroleum-based polyester terephthalate for the preparation of films, fibers, and other plastic materials.12 L-Lactide is an important intermediate in the industrial production of high molar mass PLLA, and the quality of L-lactide is a crucial parameter for controlling the properties of the resulting PLLA products. Obviously, it is necessary that L-lactide should possess a high chemical and optical purity. Therefore, the synthesis of enantiomerically pure L-lactide is of practical importance for the preparation of high-Mw PLLA.L-Lactide can be obtained by the thermal degradation of oligomeric PLLA (O-PLLA).13 A series of Sn, Al, Ti, Zn and Zr compounds have been used as catalysts for producing lactide from PLA oligomer.14,15 This process inevitably undergoes racemization because of an amply high temperature and an amply long reaction time.16 Yoo et al.17 demonstrated that the racemization of lactide occured via deprotonation due to its high sensitivity to weak bases, and this process was accelerated by increased temperature. Idage et al.9 synthesized L-lactide with a yield of about 98% using zinc and tin metal catalysts of less than 150 micron particle size, and the crude L-lactide was further purified through an extremely complicated process. The purification step mainly consisted of recrystallization of crude lactide from boiling anhydrous toluene, filtration and repeatedly washing of lactide crystals. Ehsani and co-workers18 carried out a comprehensive study of the lactide synthesis optimization. They showed that increasing temperature resulted in higher amounts of impurities in the crude lactide. Huang et al.19 successfully conducted the green synthesis of the enantiomerically pure L-lactide and D-lactide using the creatinine-guanidinium catalyst. Synthesis of L-lactide using an onium salt catalyst was reported by Ishijima et al.20 More various catalyst systems have been investigated to maximize the yield in synthesis of lactide, while minimizing the racemization and simplifying the purification. However, few studies have been focused on the ionic liquids (ILs) catalyst that can activate PLA oligomer.
Recently, halometallate ionic liquids (ILs) are frequently introduced as relatively clean and promising catalysts and solvents, because of their unique properties such as high thermal stability, potential recoverability and fine control over physical and chemical properties by wide selection of the halometallate anions.21–23 In particular, many studies on polymerization progresses catalyzed by ILs have been done with high catalytic activities.24–26 Nevertheless, little attention has been paid to the study of the synthesis of lactide from PLA oligomer using ILs as catalysts. Kim et al.27 prepared lactide using IL only as a solvent, resulting in a lower reaction temperature and higher mobility of the reactant. In our attempt to search for catalysts used in the depolymerization of O-PLLA, we speculate that chlorostannate(II) ionic liquids may be good candidates for three reasons. Firstly, the chlorostannate(II) ionic liquid can behave as a Lewis acid21 and some Lewis acids are catalysts for O-PLLA depolymerization. Secondly, the depolymerization of O-PLLA into L-lactide is normally carried out under high temperature and low pressure, which requires catalysts with high thermal stabilities. Chlorometallate ionic liquids are generally thermally stable and less volatile,28 which means that they may be excellent catalysts for depolymerization. Thirdly, chlorostannate(II) ionic liquids are more inexpensive than those incorporating gallium(III) or indium(III) and less sensitive toward moisture than those based on aluminum(III) and gallium(III).29 In this study, the imidazolium-based ionic liquids incorporating stannum(II) were successfully prepared. The structure, thermal stability, and acidity of these synthesized ILs were experimentally determined and systematically analyzed. Then the depolymerization of O-PLLA was carried out using these ILs as catalysts for the first time, to produce L-lactide with high optical purity. A possible catalysis mechanism was proposed. We also investigated the effects of various reaction parameters, such as temperature, reaction time, pressure and mole ratio of catalyst/O-PPLA. Furthermore, the residue polymers of depolymerization were reutilized for the resynthesis of L-lactide.
Experimental section
Materials
L-Lactic acid (L-LA) as a 90 wt% aqueous solution was purchased from Musashino Chemical (China) Company. Tin(II) chloride (Aladdin, water-free for synthesis, ≥99.0%), n-methylimidazole (Aladdin, ≥99.0%) and pyridine (Aladdin, HPLC, ≥99.9%) were used without any further purification. 1-Chlorobutane (AR), acetonitrile (HPLC), hydrochloric acid (AR), ethanol (AR) were purchased from Jiangtian Co. Ltd. and used as received.Preparation of ionic liquids
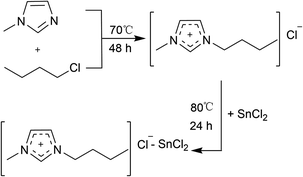
The chlorostannate(II) ionic liquids were synthesized according to the published literature,29,30 and these ILs are shown in Scheme 1.In this work, the molar fraction (χ) of SnCl2 in the synthesized ILs is defined as
 | (1) |
Analytical methods of ILs
[Bmim]Cl–SnCl2 systems were identified by 1H NMR spectrometer. NMR spectra were obtained on a Varian INOVA-500 spectrometer with DMSO as a solvent and TMS as an internal standard. The thermal stability of the [Bmim]Cl and [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) were determined by thermogravimetric analysis (TGA) at a heating rate of 10 K min−1 under nitrogen atmosphere from 25 to 500 °C. Raman spectra of the needle-shaped precipitate in [Bmim]Cl–SnCl2 (xSnCl2 = 0.67) were recorded using a RENISHAW InVia Reflex Raman spectrometer, with a 785 nm focused laser beam.Evaluation of acidity of ILs
Yang and Kou31 have determined the acidity of ILs by means of an IR spectroscopic probe, wherein pyridine was used as a probe molecule for determination of the Lewis and Brønsted acidity of ILs and ethanenitrile, a weaker Lewis base than pyridine, was also used to measure the Lewis acidity of ILs. Acidity evaluation was carried out according to the following procedure: the samples were prepared by respectively adding pyridine and ethanenitrile to ILs in a given volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and then these samples were analyzed by IR spectroscopy. The spectra were also recorded on the Bruker TENSON 27 FT-IR spectrometer. All spectra were acquired at a 4 cm−1 resolution with a total of 64 scans per spectrum.
3, and then these samples were analyzed by IR spectroscopy. The spectra were also recorded on the Bruker TENSON 27 FT-IR spectrometer. All spectra were acquired at a 4 cm−1 resolution with a total of 64 scans per spectrum.
Reaction procedure and analysis
 | (2) |
Recycling of PLLA residue for L-lactide resynthesis
The PLLA residue (PLLAr) was formed after lactide synthesis by the prepolymer route. A mixture of L-lactic acid (71.50 g, 0.71 mol, 90 wt% aqueous solution) and PLLAr (24.45 g, 0.34 mol) was heated at 120 °C under nitrogen atmosphere. The hydrolytic degradation was conducted for 24 h under continuous stirring to obtain monomeric lactic acid (DP < 2, calculated by comparing the integral areas of 1H NMR signals). Then, the L-lactide was resynthesized from the prepolymerization reaction step, according to Scheme 2.Results and discussion
Characterizations of the ionic liquids
As demonstrated in Fig. 1a, the δC2–H signals are strongly shifted far upfield when compared to the chemical shifts of other protons. This demonstrates that the C-2 proton is the most sensitive towards variations of hydrogen -bond-accepting ability of the anions. The δC2–H for neat [Bmim]Cl is very high (10.27 ppm), which indicates that there is much more hydrogen bonding to the chloride anion than complex anions. Moving towards higher xSnCl2 values (xSnCl2 < 0.50), the δC2–H signals are shifted to higher fields, but the chemical shifts for xSnCl2 = 0.60–0.67 appear to be plateaued at around 9.1 ppm, as shown in Fig. 1b. Upon addition of tin(II) chloride, changes of δC2–H signals disclose that the strength of the cation–anion interactions decreases. This could be interpreted in terms of the formation of complex anion clusters. To the best of our knowledge, the existence of two chlorostannate(II) anions: [SnCl3]−and [Sn2Cl5]−, has been demonstrated.29 For xSnCl2 = 0.50, the δC2–H signal is shifted to 9.37 ppm, since that electron-rich anions ([SnCl3]−) are present, resulting in shielding of the H nucleus. The progressive addition of tin(II) chloride led to a gradual decrease of the chemical shift for xSnCl2 = 0.50–0.60. Above this concentration (xSnCl2 = 0.60), it is possible that the [Sn2Cl5]− dimer is formed, which leads to further weakening of cation–anion interactions, because of charge distribution of a single negative charge over a greater number of electronegative centres. But there are not enough dimers present for H-bonding to each imidazolium cation in [Bmim]Cl–SnCl2 systems, due to the formation of tin(II) chloride precipitate for xSnCl2 > 0.63 samples. It appears that the composition of [Bmim]Cl–SnCl2 systems for xSnCl2 ≥ 0.63 remained unchanged. In a word, for xSnCl2 = 0.50, only [SnCl3]− anion is present in [Bmim]Cl–SnCl2 systems; for xSnCl2 > 0.50, there is two kinds of chlorostannate(II) anions: [SnCl3]−and [Sn2Cl5]−. It is worth noting that [Bmim]Cl–SnCl2 systems based solely on [Sn2Cl5]− clusters as anions are unstable and inexistent at room temperature.
Evaluation of acidity of the ionic liquids
As shown in Fig. 3, the bands of acetonitrile/[Bmim]Cl and acetonitrile/[Bmim]Cl–SnCl2 (xSnCl2 = 0.33) were nearly the same as that of acetonitrile. They showed two characteristic bands at approximately 2295 and 2250 cm−1, originating from its CN stretching vibrations, indicative of the lack of Lewis acidity of [Bmim]Cl and [Bmim]Cl–SnCl2 (xSnCl2 = 0.33). However, in the spectra of acetonitrile/[Bmim]Cl–SnCl2 (xSnCl2 = 0.50), acetonitrile/[Bmim]Cl–SnCl2 (xSnCl2 = 0.60) and acetonitrile/[Bmim]Cl–SnCl2 (xSnCl2 = 0.63), new absorption peaks at around 2330 cm−1 appeared. The new absorption peak was the characteristic absorption peak of the CN-Lewis complex, indicating that [Bmim]Cl–SnCl2 (xSnCl2 = 0.50), [Bmim]Cl–SnCl2 (xSnCl2 = 0.60) and [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) had Lewis acidity. Moreover, when xSnCl2 > 0.50, a monotonic blue shift of new bands was observed with increasing value of xSnCl2, corresponding to an increase in Lewis acidic strength.
L-Lactide synthesis
The sample of O-PLLA, with the average degree of polymerization of 12 (see Fig. S2 in ESI†), was synthesized. When the depolymerization reaction was carried out using O-PLLA (DP = 12), the effect of different catalyst systems on the yield of crude lactide was studied (see Fig. 5). The distillate solid is defined as crude lactide, containing D-lactide, L-lactide, meso-lactide, and other impurities such as lactic acid, lactoyl lactic acid and other acyclic. The yield is expressed by eqn (3):
 | (3) |
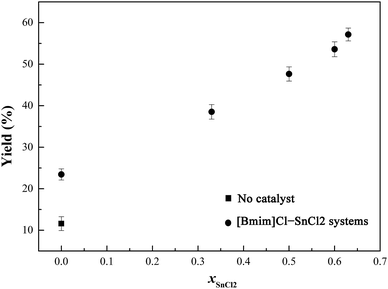 | ||
| Fig. 5 Effect of [Bmim]Cl–SnCl2 catalyst systems on the yield of the crude lactide. Reaction conditions: 200 °C, 10 torr, 2 h, O-PLLA (DP = 12), 300 rpm, catalyst 0.1 mol% relative to O-PLLA. | ||
As can be seen from Fig. 5, using the catalyst multiplied the yield of crude lactide, and as to the [Bmim]Cl–SnCl2 catalyst systems, the crude lactide was obtained in moderate to good yield (39%–57%). Meanwhile, the yield increased significantly as the molar fraction of SnCl2 increased from 0 to 0.63. This was mainly because with an increased amount of SnCl2, the acidity of the ionic liquids became stronger. These catalysts were uniformly dispersed in the mixture and sufficiently contacted with the reactants, because they were miscible with O-PLLA at a catalytic amount throughout the depolymerization process. Synthesis of L-lactide is also summarized in Table 1, using SnCl2, [Bmim]Cl, or [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) as a catalyst, respectively. D,L-lactide means a minute amount of D-lactide in addition to L-lactide. The fractional amount of D,L-lactide in crude lactide could be calculated by comparing the integral areas of 1H NMR signals (see Fig. S3 in ESI†). The results (Table 1) indicated that D,L-lactide fraction was significantly increased by using IL catalyst. It meaned that the presence of ILs reduced or eliminated the negative impact of side reactions. It is known that, meso-lactide, lactic acid and acyclic oligomers are more soluble in water than L-lactide. Thus, water-washing method was conducted to obtain the pure lactide.
| No. | Catalyst | Lactide synthesis | Residue | ||
|---|---|---|---|---|---|
| D,L-lactideb | OPc | DPd | Iso.e | ||
| a 200 °C, 10 torr, 2 h, O-PLLA (DP = 12), 300 rpm, catalyst 0.1 mol% relative to O-PLLA.b Calculated by 1H NMR. (see Fig. S3 in ESI).c Measured with an automatic polarimeter.d Measured with 1H NMR. (see Fig. S5 in ESI).e Isotacticity measured with 13C NMR. | |||||
| 1 | SnCl2 | 77.5% | 90.0% | 56 | 78.5% |
| 2 | [Bmim]Cl | 91.2% | 98.4% | 52 | 95.4% |
| 3 | [Bmim]Cl–SnCl2(xSnCl2 = 0.63) | 97.9% | 99.9% | 49 | 99.1% |
When using [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) as a catalyst, the optical purity of purified lactide was 99.9%, which was much higher than using SnCl2. As for the isotacticity of residues, the same situation occurred. This was probably because the formation of the complex clusters (e.g., [SnCl3]−, [Sn2Cl5]−) enhanced the acidity of the anion, then decreased the degree of the racemization that usually occurred via the α-proton abstraction.17
It is clear that the synthetic system of L-lactide involves two reaction equilibria: depolymerization equilibrium and polycondensation equilibrium of partial O-PLLA molecules. The L-lactide is produced by the depolymerization of O-PLLA, which is actually an intra-molecular transesterification reaction.37–39 Based on the structure of ILs (as shown in Scheme 1), we proposed a possible mechanism, as illustrated in Scheme 3. The imidazolium cation forms a tight association with the carbonyl oxygen so as to enhance the electrophilicity of the carbonyl-carbon. Then, the attack of a hydroxyl terminal of O-PLLA molecule upon the carbonyl-carbon followed by the intra-molecular transesterification leads to the formation of six-membered cyclic ester. It should be mentioned that, the counter anion of the imidazolium cation is the terminal carboxylate in the O-PLLA chain and the steric hindrance of the terminal carboxylate group is very high. By this means, the chance of the possible racemization via α-proton abstraction17,19 is greatly reduced. The isotacticity of the residual polymers has proved this point (for [Bmim]Cl–SnCl2 (xSnCl2 = 0.63), Iso. = 99.1%; for SnCl2, Iso. = 78.5%), as listed in Table 1.
 | ||
| Scheme 3 Possible mechanism of depolymerization – polycondensation behavior of O-PLLA catalyzed by 1-butyl-3-methylimidazolium ionic liquids. | ||
According to the above mechanism, both the strength of the cation–anion interaction and the Lewis acidic strength of ILs play important roles in the catalytic activity. The strong cation–anion interaction hinders the imidazolium from getting close to the O-PLLA, thus reducing the catalytic activity. ILs incorporating stannum(II) weaken the cation–anion interaction and enhance the Lewis acidity of the imidazolium, resulting in higher catalytic activity. These considerations are in agreement with our experimental results.
Effects of various reaction parameters
The effects of various reaction parameters, such as temperature, reaction time, pressure and mole ratio of catalyst/O-PPLA, were investigated in detail.Fig. 6 displays the effect of the reaction temperature on the yield of crude lactide in the temperature range of 180–240 °C. As the reaction temperature increased, the yield went up gradually due to the fact that the depolymerization reaction rate increased with increasing temperature. As far as we know, the side reactions such as racemization, elimination or formation of the ether derivatives, are accelerated by increased temperature.17,20 In order to ensure the chemical and optical purity of L-lactide, the results indicated that 200 °C was an appropriate reaction temperature for [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) ionic liquid.
 | ||
| Fig. 6 Variation of yield% as a function of reaction temperature. Reaction conditions: 10 torr, 2 h, O-PLLA (DP = 12), 300 rpm, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) 0.1 mol% relative to O-PLLA. | ||
The effect of reaction time on crude lactide yield was studied by varying the reaction time from 0 to 4 h. As illustrated in Fig. 7, the yield obviously increased with increasing of reaction time from 0 to 2 h. However, a slight increase in the yield was observed when the reaction time was increased from 2–4 h. Hence, we chose 2 h as the suitable reaction time in the subsequent experiments.
 | ||
| Fig. 7 Variation of yield% as a function of reaction time. Reaction conditions: 200 °C, 10 torr, O-PLLA (DP = 12), 300 rpm, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) 0.1 mol% relative to O-PLLA. | ||
Fig. 8 shows the variation of crude lactide yield as a function of pressure. The yield increased from 26 to 57% as the pressure was decreased from 100 to 10 torr. After the lactide was formed, it was distilled immediately at the lower pressure, thus increasing the conversion.
 | ||
| Fig. 8 Variation of yield% as a function of pressure. Reaction conditions: 200 °C, 2 h, O-PLLA (DP = 12), 300 rpm, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) 0.1 mol% relative to O-PLLA. | ||
Fig. 9 shows the influence of the catalyst concentration on the yield of crude lactide. The yield increased rapidly from 48 to 60% as the mole ratio of [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) to O-PLLA increased from 0.025 to 0.1. The higher yield obtained at a higher catalyst concentration may have been due to the higher reaction kinetics. However, the further increase in the mole ratio would not cause an obvious increase in the yield of crude lactide. Therefore, the optimal mole ratio was 0.1.
 | ||
| Fig. 9 Variation of yield% as a function of mole ratio of [Bmim]Cl–SnCl2 (xSnCl2 = 0.63)/O-PPLA. Reaction conditions: 200 °C, 10 torr, 2 h, O-PLLA (DP = 12), 300 rpm. | ||
Recycle of PLLAr for L-lactide resynthesis
During the process of synthesizing L-lactide by the prepolymer route, the high-Mw oligomer residue is inevitably formed. The reasonable recycle of these residual polymers is important in both academia and industry as viewed from the cost reduction and environment protection. When using [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) as a catalyst, the residual polymer of DP = 49 was formed and it possessed high isotacticity of 99.1% that was much higher than using the conventional SnCl2 catalyst (Table 1). Obviously, this makes it possible to realize the reutilization of these residues for the resynthesis of L-lactide (Table 2).| Rec.b | Hydrolysis step | Prepolymerization step | Depolymerization step | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-LA replenished | O-PLLA (DP = 12) | L-Lactide | Residue | |||||||||
| wt (g) | molc | wt (g) | molc | Oligomer yield | wt (g) | molc | Yieldd | Yielde | wt (g) | DPf | Iso.g | |
| a 200 °C, 10 torr, 2 h, 300 rpm, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) 0.1 mol% relative to O-PLLA.b Recycle time.c Based on lactic acid unit.d Base on O-PLLA.e Base on L-LA replenished.f Measured with 1H NMR.g Isotacticity measured with 13C NMR. | ||||||||||||
| 0 | 100.00 | 1.00 | 67.76 | 0.92 | 92.2% | 38.76 | 0.54 | 57.2% | 24.45 | 49 | 99.1% | |
| 1 | 71.50 | 0.71 | 72.87 | 0.99 | 94.2% | 42.78 | 0.59 | 58.7% | 83.1% | 26.80 | 54 | 98.8% |
| 2 | 78.55 | 0.78 | 79.12 | 1.08 | 93.1% | 44.90 | 0.62 | 56.7% | 79.4% | 26.15 | 46 | 98.6% |
| 3 | 76.32 | 0.76 | 76.57 | 1.04 | 92.6% | 44.00 | 0.61 | 57.5% | 80.1% | 25.48 | 52 | 98.5% |
The residual polymer was hydrolyzed with aqueous lactic acid (90 wt%) to regenerate monomeric lactic acid. The amount of water in the added lactic acid is 1.2 mole equivalent to hydrolyze PLLAr. The hydrolysate was successively subjected to oligomerization and depolymerization to resynthesize L-lactide. As shown in Table 2, the reiterative L-lactide synthesis proceeded as efficiently as the first run to give stable yields of 56.7%–58.7% (Based on O-PLLA). The loss in the oligomer yield was due to vaporization of the unreacted L-lactic acid and oligomer with low molecular weight less than 500 g mol−1. Notably, the [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) catalyst was entirely contained in the high-Mw oligomer residues because of its high thermal stability (Td = 390 °C). Thus, the reiterative depolymerization was conducted without further addition of the catalyst. Under the same reaction conditions, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) catalyst showed no notable loss of activity up to three cycles. It was worth noting that, in this process, the yield calculated on the basis of the replenished L-lactic acid after the second cycle reached up to 80–83%, which indicated the practicability of the present recycle process. In addition, the isotacticity of PLLAr was maintained at a high level during the recycle process. Hence, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) is thought to be an efficient and recyclable catalyst for L-lactide production by the prepolymer route.
Conclusions
In this study, it is the first time that chlorostannate(II) ionic liquids have been used as catalyst for depolymerization of O-PLLA into L-lactide with high chemical and optical purity. The catalytic activity increases with the more ratio of SnCl2 increase in the ILs. Meanwhile, the experimental results showed that the optimum reaction conditions (200 °C, 10 torr, 2 h, 300 rpm, [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) 0.1 mol% relative to O-PLLA) were obtained, resulting in that L-lactide is white crystalline powder with little impurity (<0.1%). Because the use of these ionic liquids minimizes the contamination of a reactive group with by-products, the use of recrystallization for purification of L-lactide is unnecessary, which would be helpful for simplifying the production process of enantiomerically pure lactide. Further, owing to a high boiling point of [Bmim]Cl–SnCl2 (xSnCl2 = 0.63), this catalyst can be easily recycled. The residue polymers of high isotacticity were utilized to resynthesize L-lactide for 3 circles without loss of the yield of lactide. Remarkably, the lactide yield reached to about 80% (based on L-LA replenished). The results show the feasibility of using [Bmim]Cl–SnCl2 (xSnCl2 = 0.63) as a catalyst for the resynthesis of high-quality L-lactide. This procedure would contribute to reduce the cost of the synthetic process of high-quality L-lactide, owing to the recycle of PLLAr.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (no. 21336007).Notes and references
- K. M. Nampoothiri, N. R. Nair and R. P. John, Bioresour. Technol., 2010, 101, 8493 CrossRef PubMed.
- A. K. Mohanty, M. Misra and G. Hinrichsen, Macromol. Mater. Eng., 2000, 276, 1 CrossRef.
- W. Groot, in Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, And Applications, ed. R. Auras, L.-T. Lim, S. E. M. Selke
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and
and![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H. Tsuji,
H. Tsuji,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) John Wiley & Sons, Inc.,
John Wiley & Sons, Inc.,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2010, ch. 1, pp. 3–16 Search PubMed.
2010, ch. 1, pp. 3–16 Search PubMed. - L. T. Lim, R. Auras and M. Rubino, Prog. Polym. Sci., 2008, 33, 820 CrossRef CAS PubMed.
- S. I. Moon, C. W. Lee, M. Miyamoto and Y. Kimura, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1673 CrossRef CAS.
- H. Li, C. H. Wang, F. Bai, J. Yue and H. G. Woo, Organometallics, 2004, 23, 1411 CrossRef CAS.
- O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147 CrossRef CAS PubMed.
- Y. Yoshiaki and A. Tomohiro, US Pat., 5502215, 1996.
- B. B. Idage and S. Swaminathan, US Pat., 20130060051A1, 2012.
- D. Garlotta, J. Polym. Environ., 2001, 9, 63 CrossRef CAS.
- S. I. Moon, C. W. Lee, I. Taniguchi, M. Miyamoto and Y. Kimura, Polymer, 2001, 42, 5059 CrossRef CAS.
- K. Hirao and H. Ohara, Polym. Rev., 2011, 51, 1 CrossRef CAS PubMed.
- Y. Liu, R. Wei, J. Wei and X. Liu, Progr. Chem., 2008, 20, 1588 CAS.
- M. Noda and H. Okuyama, Chem. Pharm. Bull., 1999, 47, 467 CrossRef CAS.
- H. Tsuji, I. Fukui, H. Daimon and K. Fujie, Polym. Degrad. Stab., 2003, 81, 501 CrossRef CAS.
- M. Noda, Prep. Biochem. Biotechnol., 1999, 29, 333 CrossRef CAS PubMed.
- D. K. Yoo, D. Kim and D. S. Lee, Macromol. Res., 2006, 14, 510 CrossRef CAS.
- M. Ehsani, K. Khodabakhshi and M. Asgari, e-Polym., 2014, 14, 353 CAS.
- W. Huang, Y. Qi, N. Cheng, X. Zong, T. Zhang, W. Jiang, H. Li and Q. Zhang, Polym. Degrad. Stab., 2014, 101, 18 CrossRef CAS PubMed.
- Y. Ishijima, Y. Maruta and A. Abiko, Chem. Lett., 2012, 41, 1456 CrossRef CAS.
- J. Estager, J. D. Holbrey and M. Swadzba-Kwasny, Chem. Soc. Rev., 2014, 43, 847 RSC.
- A. W. Taylor, S. Men, C. J. Clarke and P. Licence, RSC Adv., 2013, 3, 9436 RSC.
- H. Iwahashi, T. Oka and A. Abiko, Chem. Lett., 2008, 37, 708 CrossRef CAS.
- W. Wang, L. Wu, Y. Huang and B. G. Li, Polym. Int., 2008, 57, 872 CrossRef CAS PubMed.
- S. Zhang, H. Lefebvre, M. Tessier and A. Fradet, Green Chem., 2011, 13, 2786 RSC.
- S. Mallakpour and M. Dinari, Iran. Polym. J., 2011, 20, 259 CAS.
- S. H. Kim, C. H. Hong, D. S. Han and J. Y. Seo, US Pat., 2014187798A1, 2014.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- M. Currie, J. Estager, P. Licence, S. Men, P. Nockemann, K. R. Seddon, M. Swadzba-Kwasny and C. Terrade, Inorg. Chem., 2013, 52, 1710 CrossRef CAS PubMed.
- P. Illner, A. Zahl, R. Puchta, N. van Eikema Hommes, P. Wasserscheid and R. van Eldik, J. Organomet. Chem., 2005, 690, 3567 CrossRef CAS PubMed.
- Y. L. Yang and Y. Kou, Chem. Commun., 2004, 226 RSC.
- P. L. Salzberg, US Pat., 2758987, 1956.
- A. Elaiwi, P. B. Hitchcock, K. R. Seddon, N. Srinivasan, Y.-M. Tan, T. Welton and J. A. Zora, J. Chem. Soc., Dalton Trans., 1995, 3467 RSC.
- J. A. McCune, P. He, M. Petkovic, F. Coleman, J. Estager, J. D. Holbrey, K. R. Seddon and M. Swadzba-Kwasny, Phys. Chem. Chem. Phys., 2014, 16, 23233 RSC.
- Z. K. Zhao, B. Yuan, W. H. Qiao, Z. S. Li, G. R. Wang and L. Cheng, J. Mol. Catal. A: Chem., 2005, 235, 74 CrossRef CAS PubMed.
- X. H. Wang, G. H. Tao, Z. Y. Zhang and Y. Kou, Chin. Chem. Lett., 2005, 16, 1563 CAS.
- T. Motoyama, T. Tsukegi, Y. Shirai, H. Nishida and T. Endo, Polym. Degrad. Stab., 2007, 92, 1350 CrossRef CAS PubMed.
- C. L. Sungyeap Hong, Mod. Chem. Appl., 2014, 02, 144 Search PubMed.
- S. A. Mulyashov, F. S. Sirovski and S. G. Beksaev, Chem. Eng. J., 2011, 176, 225 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Raman spectra (Fig. S1), molecular weight determination of O-PLLA (Fig. S2), calculation the fractional amount of D,L-lactide (Fig. S3), isotacticity measured with 13C NMR (Fig. S4), synthesis of PLLA by using ILs catalysts (Table S1), 1H NMR spectra of PLLA residues (Fig. S5). See DOI: 10.1039/c5ra05073a |
| This journal is © The Royal Society of Chemistry 2015 |