Catalytic cleavage of C–O linkages in benzyl phenyl ether assisted by microwave heating†
Jun Hu,
Dekui Shen,
Shiliang Wu,
Huiyan Zhang and
Rui Xiao*
Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, PR China. E-mail: ruixiao@seu.edu.cn
First published on 11th May 2015
Abstract
The microwave-assisted cleavage of C–O linkages in benzyl phenyl ether was investigated at 200 °C in the presence of ZrP or SBA-15 doped with or without metal catalyst (Pd/C or Ru/C). The total yield of phenol and benzyl alcohol from benzyl phenyl ether depolymerization with ZrP was 26.91%, while the yield of phenol reached 26.11% for that with Pd/C. Hybrid catalysts performed effectively for promoting the formation of phenol. A conversion of 85.70% was achieved with ZrP–Pd/C catalyst, and the selectivity to phenol reached 47.32%. The highest yield of phenol was obtained at 1 h, and repolymerization was remarkable at longer reaction time. Based on the reaction using phenol and benzyl alcohol as the reactants, benzyl phenyl ether might be decomposed into phenol and benzyl alcohol following with the repolymerization producing thermally stable polymers in the presence of Pd.
1. Introduction
Biomass is the only renewable resource for the production of liquid fuels and chemicals.1 As one of the three main components of biomass, lignin is a potential feedstock to be converted into aromatics and phenolic compounds due to its abundant methoxylated phenyl-propane units connected with C–O and C–C bonds.2–4 Lignin can be converted by various thermal–chemical ways and hydrothermal is a promising way to produce liquid products.5–9 Extensive studies have been carried out on the hydrothermal degradation of various lignins, and most of these studies were conducted under electric heating.5,10–13These days, microwave heating have drawn increasingly attention in terms of biomass and lignin conversion, due to its cost-effective heating compared with electric heating.14–18 Despite these work, the degradation mechanism of lignin is not clear yet. One main reason is that the complex structure of real lignin usually lead to a wide range of product distribution.2 In this work, benzyl phenyl ether is selected as the model compound to give insight into the cleavage mechanism of α-O-4 bond which is one of the main ether bonds in real lignin.19–22
Catalyst plays a vital role in the conversion of lignin. Roberts et al. reported that the conversion of benzyl phenyl ether without catalyst took 4 h to reach 91% at 270 °C.19 When catalysts were used, efficient cleavage of C–O bonds can be fulfilled under mild conditions. He et al. reported that the conversion of benzyl phenyl ether with Ni/HZSM-5 reached about 97% after 100 min at 250 °C.20,21 Park et al. investigated the degradation of benzyl phenyl ether with Pd catalyst supported on cesium-exchanged heteropolyacid, reporting a conversion of 78% after 1 h at 200 °C, with phenol, benzene and toluene as the main products.23 These literatures confirmed that catalyst is important for promoting the conversion.
The target of this work is to investigate the microwave-assisted cleavage of C–O linkages in benzyl phenyl ether catalyzed by ZrP or SBA-15 doped with or without metal catalyst (Pd/C or Ru/C). As a solid catalyst, ZrP performs well for ether bonds cleavage in conversion of xylose and glucose.24,25 SBA-15 is widely used as catalyst support due to its large surface area and uniform tubular mesopores.26 Metal catalysts of Pd/C and Ru/C were reported as good catalysts for lignin and biomass conversion.27,28 The reactions of phenol and benzyl alcohol which are the possible intermediate products were carried out to further study the conversion pathway of benzyl phenyl ether.
2. Materials and methods
2.1. Materials
The solvents used in the study were purchased from Nanjing Reagent Co. and used as received. 5 wt% Ru/C catalyst and 5 wt% Pd/C catalyst were purchased from Aladdin. SBA-15 was purchased from Nanjing XFNANO Materials Tech Co., Ltd. Benzyl phenyl ether (>98%, GC assay) was purchased from Tokyo Chemical Industry Co., Ltd. (TCI). NH4H2PO4 and ZrOCl2·8H2O were purchased from Sinopharm chemical reagent Co., Ltd.2.2. Preparation and characterization of catalysts
The zirconium phosphate catalyst (ZrP) was prepared according to the published work.29 In brief, NH4H2PO4 (1 mol L−1, 200 mL) was added to ZrOCl2·8H2O (1 mol L−1, 100 mL) at the P/Zr mole ratio of 2. The solution was stirred for 30 min. The precipitate was filtered and washed with deionized water until the pH of 5, dried at 100 °C overnight, and then calcined at 400 °C for 4 h in air.Ammonia temperature-programmed desorption (NH3-TPD) was carried out on a quartz U-tube apparatus. The 40–60 mesh sample (0.1 g) was firstly degassed in a He stream at 400 °C for 30 min. Then the temperature was cooled down to 100 °C and 5% NH3/N2 was used for adsorption under a flow of 20 mL min−1 for 0.5 h. After that, pure He was switched to remove any physically adsorbed NH3 for 0.5 h under the same temperature. Desorption of NH3 was performed from 100 °C to 700 °C at a rate of 10 °C min−1 and the temperature was held at 700 °C for another 1 h. The desorbed NH3 was measured using a gas chromatograph (GC-SP6890) with a thermal conductivity detector (TCD). XRD was performed on a D/max rC X-ray diffractometer using Cu Kα radiation from 5° to 80° with a step of 0.02° per s.
2.3. Depolymerization of lignin
The hydrothermal degradation of benzyl phenyl ether was conducted in a microwave reaction system (MDS-6, Sineo, China). The 70 mL vessel was made of Teflon, with a maximum working condition of 4 MPa and 220 °C. In a typical run, 0.2 g benzyl phenyl ether and 0.1 g ZrP or SBA-15 doped with or without 0.1 g Pd/C or Ru/C were loaded into the reactor vessel with 15 mL deionized water. Then the reactor was heated by microwave irradiation to 200 °C in three steps (three minutes to 160 °C, three minutes to 180 °C and three minutes to 200 °C). After keeping at 200 °C for a desired time, the reactor was cooled to room temperature by forced-air. Each reaction condition was repeated at least twice.After reaching the room temperature, the reactor was opened, and all liquid and solid products were collected. The reactor was rinsed with ethyl acetate, then the ethyl acetate was added into the collected products. The liquid product was separated from the solid catalysts by filtration. The obtained liquid product was then extracted with ethyl acetate three times, and the ethyl acetate extracted liquid product was calibrated to 100 mL by adding ethyl acetate for further GC and GC MS analysis.
2.4. Characterization of the products
The liquid products were identified by GC-MS (Agilent 7890A-5975C). The condition of GC MS was set to be: the injector temperature was kept at 300 °C; the chromatographic separation was performed with a HP-5 MS capillary column; the oven temperature was programmed from 40 °C (3 min) to 180 °C (2 min) with 5 °C min−1 heating rate, and then to 280 °C (2 min) with 10 °C min−1 heating rate; the mass spectra were operated in EI mode at 70 eV. The mass spectra were obtained from m/z 50 to 650. The chromatographic peaks were identified according to the NIST MS library and previously published work.30,31 The yield of product i (mass yield, Yi) was quantified by GC (Shimadzu 2014) with an AT.SE 54 capillary column and a FID detector, and calculated as:
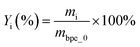 | (1) |
The conversion (C) was determined by the weight difference of benzyl phenyl ether before and after reaction, and was calculated as:
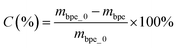 | (2) |
The selectivity (Si) was calculated as:
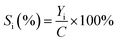 | (3) |
3. Results and discussion
3.1. Characterization of the catalysts
Fig. 1 shows the NH3-TPD desorption profiles of ZrP and SBA-15. One strong broad desorption peak could be observed for ZrP from 570 to 660 °C, indicating the existence of strong acid site. SBA-15 was reported with no acid sites. However, one small broad desorption peak could be found around 550 °C for SBA-15 in the present work. The weak peak may be attributed to the existence of silanols.32 The absolute peak area of the NH3-TPD desorption profile is considered to be linear with the amount of its acid sites.25 Calculated based on the peak area, ZrP possessed the acid sites of 0.2175 mmol g−1.The X-ray diffractogram (XRD) patterns of ZrP, SBA-15, Pd/C and Ru/C are shown in Fig. 2. For ZrP catalyst, two broad peaks in 2θ range of 15–40° and 40–70° indicated its amorphous nature, which was consistent with the published work.24,25 SBA-15 also exhibited amorphous structure with one strong broad peak in the range of 2θ from 20° to 40°. For the two metal catalysts supported on activated carbon, one board peak around 24° could be observed and this peak corresponded to the amorphous carbon. The Ru crystallite peak at 2θ = 44° and Pd crystallite peak at 2θ = 39.34° were very weak, indicating that both Ru and Pd particles were well dispersed on the support (particle size less than 5 nm).33,34
3.2. Effect of temperature
Fig. 3 is the product yields of benzyl phenyl ether at different temperatures without catalyst. The main products were phenol and benzyl alcohol. Only 0.10% and 0.38% yield of phenol were obtained at 150 °C and 180 °C, respectively. At 200 °C, the yields of phenol and benzyl alcohol reached 4.07% and 2.74%, respectively. Degradation at higher temperature cannot be investigated in this system because of the working condition limitation. | ||
| Fig. 3 Product yields of benzyl phenyl ether without catalyst. Reaction conditions: 0.2 g benzyl phenyl ether, 15 mL H2O, 1 h. | ||
3.3. Effect of catalyst species
Benzyl phenyl ether can be partially degraded at 200 °C without catalyst, but the product yields were low. Therefore, ZrP, SBA-15, Pd/C and Ru/C were screened to promote the conversion. The conversions of benzyl phenyl ether with different catalysts are given in Table 1. The conversion with the treatment of ZrP was 59.93%. While the conversion with SBA-15 was only 45.27%. The presence of metal catalysts resulted in higher conversions. Conversions with Pd/C and Ru/C were 85.51% and 72.66% respectively (Table 1).| Entry | Catalyst | Conversion (%) | Toluene | Phenol | Benzyl alcohol | |||
|---|---|---|---|---|---|---|---|---|
| Yield (%) | Selectivity (%) | Yield (%) | Selectivity (%) | Yield (%) | Selectivity (%) | |||
| a Reaction conditions: 0.2 g benzyl phenyl ether, 0.1 g catalyst, 15 mL H2O, 200 °C. | ||||||||
| 1 | ZrP | 59.93 | 0.00 | 0.00 | 13.29 | 22.18 | 13.62 | 22.73 |
| 2 | SBA-15 | 45.27 | 0.00 | 0.00 | 8.59 | 18.98 | 5.33 | 11.77 |
| 3 | Pd/C | 85.51 | 2.75 | 3.18 | 26.11 | 30.18 | 0.00 | 0.00 |
| 4 | Ru/C | 72.66 | 0.00 | 0.00 | 8.07 | 11.10 | 2.68 | 3.69 |
As listed in Table 1, higher product yields were obtained with all catalysts compared to that without catalyst. The main products identified by GC MS were phenol, benzyl alcohol and toluene. Roberts et al. studied the products of benzyl phenyl ether under electric heating with LDI-TOF/MS, and reported that dimers and trimers could also be found in the products.19 The highest yields of phenol (13.29%) and benzyl alcohol (13.62%) were obtained with ZrP. Only 8.59% yield of phenol and 5.33% yield of benzyl alcohol were produced over SBA-15. For the metal catalysts, 8.07% yield of phenol and 2.68% yield of benzyl alcohol were obtained with the application of Ru/C. Although no benzyl alcohol was produced over Pd/C, the yield of phenol reached the highest value of 26.11% (Table 1).
3.4. Effect of hybrid catalysts
ZrP or SBA-15 was mixed with Ru/C or Pd/C to investigate the performance of hybrid catalysts, and the conversions with different hybrid catalysts are given in Table 2. Low conversion of 47.87% and 50.02% were obtained over SBA-15–Ru/C and ZrP–Ru/C, respectively. The application of ZrP–Pd/C resulted in the highest conversion of 85.7%.| Entry | Catalyst | Conversion (%) | Toluene | Phenol | Benzyl alcohol | |||
|---|---|---|---|---|---|---|---|---|
| Yield (%) | Selectivity (%) | Yield (%) | Selectivity (%) | Yield (%) | Selectivity (%) | |||
| a Reaction conditions: 0.2 g benzyl phenyl ether, 0.1 g SBA-15 or ZrP catalyst and 0.1 g metal catalyst, 15 mL H2O, 200 °C. | ||||||||
| 1 | SBA–15-Ru/C | 47.87 | 0.10 | 0.21 | 7.80 | 16.30 | 0.95 | 1.98 |
| 2 | SBA–15-Pd/C | 78.76 | 2.11 | 2.68 | 28.55 | 36.24 | 0.00 | 0.00 |
| 3 | ZrP–Ru/C | 50.02 | 0.35 | 0.70 | 14.26 | 28.52 | 3.78 | 7.56 |
| 4 | ZrP–Pd/C | 85.70 | 0.96 | 1.12 | 40.55 | 47.32 | 0.00 | 0.00 |
The yields of phenol and benzyl alcohol over SBA-15–Ru/C were 7.8% and 0.95%. And the yields of these two products over ZrP–Ru/C were 14.26% and 3.78%, respectively. The low yields with Ru/C as the metal catalyst indicates that the promotion of Ru/C is not as strong as Pd/C. The yield of phenol with the treatment of SBA-15–Pd/C was 28.55%. 2.11% yield of toluene was identified as well, while no benzyl alcohol was identified. When ZrP and Pd/C were used as the hybrid catalyst, 40.55% yield of phenol was obtained with the selectivity of 47.95%. The yield of phenol over ZrP–Pd/C was higher than that using ZrP (13.29%) or Pd/C (26.11%).
The hydrothermal stability of catalyst in aqueous phase condition is an important aspect. The X-ray diffractogram (XRD) patterns of spent ZrP and spent Pd/C are shown in Fig. S1.† The pattern of spent ZrP showed no difference compared to that of fresh ZrP, showing that ZrP was stable in terms of the crystalline structure in aqueous condition. The pattern of Pd/C also showed a typical amorphous structure of activated carbon without sharp peak of Pd, indicating that the well dispersion of Pd particles remained in the aqueous condition. NH3-TPD desorption revealed that the acid sites of ZrP decreased slightly to 0.1575 mmol g−1 after the reaction. Liao et al. observed that catalytic performance of ZrP decreased slightly for conversion of cellulose after the first use.24 They claimed that this phenomenon was attributed to P leaching under the hydrothermal reaction condition, and P leaching only took place in the first run.
3.5. Effect of reaction time
The effect of reaction time was investigated with ZrP–Pd/C catalyst at 200 °C. The conversion of benzyl phenyl ether and the product yields at 0–4 h were shown in Fig. 4. At 0 h, 41.06% conversion was observed. With the reaction time increasing, the conversion increased, reaching 85.70% at 1 h, and 99.23% at 4 h. | ||
| Fig. 4 Product yield and selectivity of benzyl phenyl ether at different reaction time. Reaction conditions: 0.2 g benzyl phenyl ether, 0.1 g ZrP and 0.1 g Pd/C, 15 mL H2O, 200 °C. | ||
The products of benzyl phenyl ether degradation were phenol and toluene. At 0 h, 6.30% phenol was obtained. The highest yield of phenol was obtained at 1 h as 41.55%. After that, the yield decreased slightly to 41.38% at 2 h and 39.97% at 4 h. Toluene reached the highest yield of 1.22% at 2 h. The results indicates that most of the benzyl phenyl ether has been consumed in 1 h with the treatment of ZrP–Pd/C. Longer reaction time to 4 h resulted in the decreasing of product yields, although the conversion is higher. This should be the result of repolymerization reaction.
3.6. Chemical pathways for the formation of primary products
According to He et al.'s report, the mole yields of phenol and benzyl alcohol formed from the depolymerization of benzyl phenyl ether catalyzed by Ni/HZSM-5 under electric heating exhibited a stoichiometry balance.21 Therefore, they proposed a hydrolysis reaction mechanism for the decomposition of benzyl phenyl ether. However, no obvious stoichiometry balance was found for phenol and benzyl alcohol in the present work. The mole yield of phenol was generally higher than that of benzyl alcohol. With Pd/C as the catalyst, benzyl alcohol was not identified in the liquid products. And the total yield of products identified by GC was lower than the calculated conversion. One possibility is that the products formed by hydrolysis reaction reacted further to form some secondary products which could not be identified by GC. To test the possibility, phenol and benzyl alcohol which might be the possible primary products were used as the reactants to investigate if some further reaction existed in our reaction system. Product yields and selectivities of phenol and/or benzyl alcohol under microwave heating are shown in Table 3.| Reactant | Catalyst | Phenol (%) | Benzyl alcohol (%) |
|---|---|---|---|
| a Calculated based on the mass amount of initial phenol.b Calculated based on the mass amount of initial benzyl alcohol. Reaction conditions: 0.2 g benzyl phenyl ether, 0.1 g ZrP and 0.1 g Pd/C, 15 mL H2O, 200 °C. | |||
| Phenol | ZrP | 97.2a | 0.00a |
| Phenol | Pd/C | 91.28a | 0.00a |
| Phenol | ZrP–Pd/C | 91.91a | 0.00a |
| Benzyl alcohol | ZrP | 4.50b | 95.56b |
| Benzyl alcohol | Pd/C | 1.77b | 0.00b |
| Benzyl alcohol | ZrP–Pd/C | 0.67b | 0.00b |
| Benzyl alcohol + phenol | ZrP | 101.83a | 96.29b |
| Benzyl alcohol + phenol | Pd/C | 109.53a | 12.24b |
| Benzyl alcohol + phenol | ZrP–Pd/C | 112.31a | 4.17b |
With phenol as the reactant and ZrP as the catalyst, 97.20% phenol was recovered, and no other products were detected by GC. When Pd/C or ZrP–Pd/C were used as the catalyst, the unreacted amount of phenol was high as well (91.28–91.91%). The results indicate that phenol is very stable even with the catalyst present.
When benzyl alcohol was used as the reactant, 95.56% benzyl alcohol remained after 1 h with ZrP as the catalyst, and 4.50% phenol was formed. This shows that benzyl alcohol is also relatively stable over ZrP. Treated with Pd/C or ZrP–Pd/C as the catalyst, only 1.77% and 0.67% phenol was produced, respectively. However, it is interesting that no unreacted benzyl alcohol or any other products was identified, showing that benzyl alcohol has all been consumed.
When phenol and benzyl alcohol were used as the reactants and ZrP used as the catalyst, the amount of phenol increased slightly, and the amount of benzyl alcohol decreased slightly to 96.29%. This is consistent with the result using phenol and benzyl alcohol as the reactant respectively. With Pd/C or ZrP–Pd/C as the catalyst, the amount of phenol increased slightly, while the amount of benzyl alcohol decreased greatly to 12.24% and 4.17% respectively, which is also consistent with the reaction using benzyl alcohol as the reactant. Therefore, it could be proposed that catalyzed by Pd/C, benzyl alcohol formed by the depolymerization of benzyl phenyl ether would undergo some further reaction forming products which could not be identified by GC, such as oligomers. If such repolymerization could be inhibited, the total yield of monomeric aromatics would be improved greatly.
3.7. Summary of the depolymerization mechanism of BPE
Various reaction mechanism has been proposed for the depolymerization of benzyl phenyl ether. One proposed mechanism was that in supercritical water, the cleavage of α-O-4 bond was proceeded via parallel hydrolysis and pyrolysis pathways to form phenol and toluene.35 Siskin et al. proposed that the radical pathway dominated the reaction above 350 °C in the aqueous phase, while at the temperature lower than 250 °C the ionic chemistry was the main pathway.36 He et al. also proposed that the C–O cleavage of benzyl phenyl ether proceeded along an ionic pathway rather than a thermal/free-radical route at 250 °C.21 Because of the presence of H2, only a slight amount of alkylation products were found in their work. Roberts et al. proposed that in the presence of alkali carbonate, the metal ion led to the formation of a cation-benzyl phenyl ether adduct, in which the ether bond was polarized and prone to be heterolytically cleaved.19Based on the previous results, possible catalyzed reaction pathway of benzyl phenyl ether was shown in Fig. 5. It could be proposed that in our case treated with ZrP, benzyl phenyl ether will firstly be hydrolyzed forming phenol and benzyl alcohol. Slight amount of benzyl alcohol will react further, resulting in the relatively lower yield of benzyl alcohol than phenol.
 | ||
| Fig. 5 Possible reaction pathway for the cleavage of benzyl phenyl ether in presence of ZrP and Pd/C. | ||
For the circumstance of Pd/C, benzyl phenyl ether will be depolymerized first forming phenol and benzyl alcohol. One possible pathway is that ether bond in benzyl phenyl ether will be polarized by forming the metal-benzyl phenyl ether, and thus was easily to be heterolytically cleaved.19 The benzyl alcohol will undergone further alkylation reaction forming undetected oligomer products.
In the presence of ZrP–Pd/C mixture catalysts, both catalysts contribute to the depolymerization. While the benzyl alcohol formed by the catalysis ZrP will be catalyzed again by Pd/C forming alkylation products.
4. Conclusion
The yield of phenol increased effectively in the presence of ZrP or SBA-15 doped with metal catalyst (Pd/C or Ru/C), and 40.55% phenol was produced over ZrP–Pd/C. As the main primary products, phenol was stable, while benzyl alcohol reacted easily forming oligomers over Pd. It is proposed that under the catalysis of ZrP–Pd/C hybrid catalyst, benzyl phenyl ether will firstly be decomposed into phenol and benzyl alcohol, and benzyl alcohol will then undergo repolymerization forming polymers.Acknowledgements
The authors greatly acknowledge the funding support from the projects supported by National Basic Research Program of China (973 Program) (Grant no. 2012CB215306 and 2011CB201505) and National Natural Science Foundation of China (Grant no. 51476034, 51476035), the Major Research Plan of the National Natural Science Foundation of China (Grant no. 91334205), and the Scientific Research Foundation of the Graduate School of Southeast University (YBJJ1325).References
- P. McKendry, Bioresour. Technol., 2002, 83, 37–46 CrossRef CAS.
- J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552 CrossRef CAS PubMed.
- Q. Song, J. Cai, J. Zhang, W. Yu, F. Wang and J. Xu, Chin. J. Catal., 2013, 34, 651–658 CrossRef CAS.
- C. Xu, R. A. D. Arancon, J. Labidi and R. Luque, Chem. Soc. Rev., 2014, 43, 7485–7500 RSC.
- S. Kang, X. Li, J. Fan and J. Chang, Renewable Sustainable Energy Rev., 2013, 27, 546–558 CrossRef CAS PubMed.
- F. Behrendt, Y. Neubauer, M. Oevermann, B. Wilmes and N. Zobel, Chem. Eng. Technol., 2008, 31, 667–677 CrossRef CAS PubMed.
- M. P. Pandey and C. S. Kim, Chem. Eng. Technol., 2011, 34, 29–41 CrossRef CAS PubMed.
- F. Jin and H. Enomoto, Energy Environ. Sci., 2011, 4, 382 CAS.
- J. Hu, D. K. Shen, S. L. Wu, H. Y. Zhang and R. Xiao, Energy Fuels, 2014, 28, 4260–4266 CrossRef CAS.
- Y. Ye, J. Fan and J. Chang, J. Anal. Appl. Pyrolysis, 2012, 94, 190–195 CrossRef CAS PubMed.
- H. Pińkowska, P. Wolak and A. Złocińska, Chem. Eng. J., 2012, 187, 410–414 CrossRef PubMed.
- J. Hu, D. K. Shen, S. L. Wu, H. Y. Zhang and R. Xiao, J. Anal. Appl. Pyrolysis, 2014, 106, 118–124 CrossRef CAS PubMed.
- Q. Song, F. Wang, J. Cai, Y. Wang, J. Zhang, W. Yu and J. Xu, Energy Environ. Sci., 2013, 6, 994–1007, 10.1039/c2ee23741e.
- A. Toledano, L. Serrano, J. Labidi, A. Pineda, A. M. Balu and R. Luque, ChemCatChem, 2013, 5, 977–985 CrossRef CAS PubMed.
- A. Toledano, L. Serrano, A. Pineda, A. A. Romero, R. Luque and J. Labidi, Appl. Catal., B, 2014, 145, 43–55 CrossRef CAS PubMed.
- H. G. Kim and Y. Park, Ind. Eng. Chem. Res., 2013, 52, 10059–10062 CrossRef CAS.
- J. Pan, J. Fu, S. Deng and X. Lu, Energy Fuels, 2014, 28, 1380–1386 CrossRef CAS.
- C. Dong, C. Feng, Q. Liu, D. Shen and R. Xiao, Bioresour. Technol., 2014, 162, 136–141 CrossRef CAS PubMed.
- V. Roberts, S. Fendt, A. A. Lemonidou, X. Li and J. A. Lercher, Appl. Catal., B, 2010, 95, 71–77 CrossRef CAS PubMed.
- J. He, C. Zhao and J. A. Lercher, J. Am. Chem. Soc., 2012, 134, 20768–20775 CrossRef CAS PubMed.
- J. He, L. Lu, C. Zhao, D. Mei and J. A. Lercher, J. Catal., 2014, 311, 41–51 CrossRef CAS PubMed.
- J. Hu, D. K. Shen, R. Xiao, S. L. Wu and H. Y. Zhang, Energy Fuels, 2013, 27, 285–293 CrossRef CAS.
- H. W. Park, S. Park, D. R. Park, J. H. Choi and I. K. Song, J. Ind. Eng. Chem., 2011, 17, 736–741 CrossRef CAS PubMed.
- Y. Liao, Q. Liu, T. Wang, J. Long, L. Ma and Q. Zhang, Green Chem., 2014, 16, 3305–3312 RSC.
- L. Cheng, X. Guo, C. Song, G. Yu, Y. Cui, N. Xue, L. Peng, X. Guo and W. Ding, RSC Adv., 2013, 3, 23228–23235 RSC.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- M. l. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2013, 114, 1827–1870 CrossRef PubMed.
- Y. Ye, Y. Zhang, J. Fan and J. Chang, Bioresour. Technol., 2012, 118, 648–651 CrossRef CAS PubMed.
- Y. T. Kim, J. A. Dumesic and G. W. Huber, J. Catal., 2013, 304, 72–85 CrossRef CAS PubMed.
- J. C. del Río, A. Gutiérrez, M. Hernando, P. Landín, J. Romero and Á. T. Martínez, J. Anal. Appl. Pyrolysis, 2005, 74, 110–115 CrossRef PubMed.
- G. Jiang, D. J. Nowakowski and A. V. Bridgwater, Energy Fuels, 2010, 24, 4470–4475 CrossRef CAS.
- A. A. Gurinov, D. Mauder, D. Akcakayiran, G. H. Findenegg and I. G. Shenderovich, ChemPhysChem, 2012, 13, 2282–2285 CrossRef CAS PubMed.
- N. Li, G. A. Tompsett, T. Zhang, J. Shi, C. E. Wyman and G. W. Huber, Green Chem., 2011, 13, 91–101 RSC.
- A. Sanna, T. P. Vispute and G. W. Huber, Appl. Catal., B, 2015, 165, 446–456 CrossRef CAS PubMed.
- C. Yokoyama, K. Nishi and S. Takahashi, Sekiyu Gakkaishi, 1997, 40, 465–473 CrossRef CAS.
- M. Siskin, G. Brons, S. N. Vaughn, A. R. Katritzky and M. Balasubramanian, Energy Fuels, 1990, 4, 488–492 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04974a |
| This journal is © The Royal Society of Chemistry 2015 |


