Synthesis of novel aryl dithian valeryl podophyllotoxin ester derivatives as potential antitubulin agents†
Hong-Yan Lin‡
,
Zi-Kang Li‡,
Hong-Wei Han,
Han-Yue Qiu,
Hong-Wei Gu,
Yong-Hua Yang* and
Xiao-Ming Wang*
State Key Laboratory of Pharmaceutical Biotechnology, NJU-NJFU Joint Institute of Plant Molecular Biology, Nanjing University, Nanjing, 210046, China. E-mail: Yangyh@nju.edu.cn; Wangxm07@nju.edu.cn; Fax: +86-25-89681381; Tel: +86-25-89681381
First published on 22nd May 2015
Abstract
Microtubules are among the most successful targets for anticancer therapy. In this study, we described the synthesis routes of the lipoyl podophyllotoxin ester derivatives and found that they can selectively inhibit the proliferation of cancer cells without damaging the non-cancer cells. Among them, L4 showed the best antiproliferation activity with IC50 = 2.68 μM against A549 cells. This effect of L4 was similar to that of CA-4 (IC50 = 2.78 μM), a typical microtubule inhibitor, but better than podophyllotoxin (IC50 = 6.57 μM) itself. Furthermore, cell cycle analysis revealed that L4 can remarkably cause cell cycle arrest in the G2/M phase in a time- and dose-dependent manner. But the effect of L4 on apoptosis inducing was not apparent enough. Moreover, confocal microscopy and western blot analysis results indicated that L4 can perturb microtubule polymerization, thus causing tumor growth inhibition.
Introduction
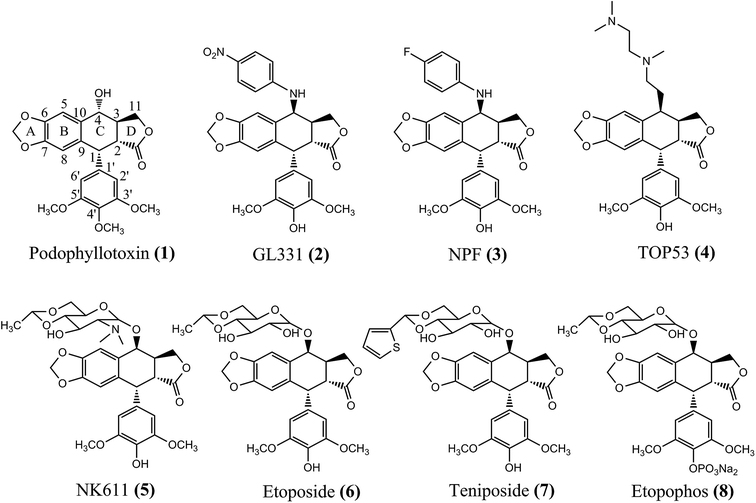
Tubulins are a universally conserved protein superfamily that carries out diverse biological roles by assembling filaments with very different architectures.1,2 Microtubules are long, hollow, cylindrical polymers composed of α- and β-tubulin dimers.3–5 They are highly dynamic structures that are regulated by polymerization and de-polymerization of tubulin, and this dynamic instability property of microtubules is important for carrying out many cellular functions.6,7 Disruption of microtubule dynamics or formation of abnormal mitotic microtubules prevents rearrangement of the microtubule network, which can induce cell cycle arrest in G2/M phase and trigger cells death.3,8,9 Additionally, the continuous mitotic division of proliferating cancer cells showed better sensitivity to the inhibition of mitosis than non-cancer cells. Based on these, microtubule has become an attractive molecular target for anticancer therapeutics.Podophyllotoxin (1, Fig. 1), a naturally occurring cyclolignan which is the main component of podophyllum resin, exhibits pronounced biological activities, especially anticancer effects through inhibiting microtubule assembly.10–12 However, the undesirable toxic side-effects of podophyllotoxin hinder its development as a new clinical anticancer agent, which have inspired the researchers to further search for new, effective anticancer agents with superior pharmacological profiles based on this scaffold.13–17 Herein, we have to note some well-known drugs, GL331 (2), NPF (3), TOP53 (4), and NK611 (5), especially the three most highly prescribed anticancer drugs in the world, etoposide (6), teniposide (7), and the water-soluble prodrug etopophos (8), that base on podophyllotoxin scaffold.13,18–20 According to previous research findings, the C-4 position of podophyllum analogs is a variable region and deserves us to further investigate. The SAR reports also suggested that the 4 position of cycloparaffin can bear bulky groups and thus has huge scope for functionalization.21
Based on the aforementioned research results, we attempted to develop new anticancer drugs by introducing α-lipoic acid derivatives into podophyllotoxin scaffold to improve its pharmacological activity and drug targeting. It is well known that the α-lipoic acid is a naturally occurring antioxidant that has been shown to possess promising anticancer activity because of its ability to preferentially induce apoptosis and inhibit proliferation of cancer cells relative to normal cells.22–24 Furthermore, in Wei's study, α-lipoic acid also plays a role as the carrier of the anticancer drug, doxorubicin during intracellular transport and trigger anticancer drug release.25 Based on these, we designed a series of aryl dithian valeryl podophyllotoxin ester derivatives and verified they have better application prospects than the parent drug, podophyllotoxin in cancer chemotherapy.
Results and discussion
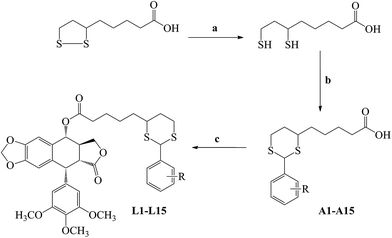
The routes to synthesizing the novel aryl dithian valeryl podophyllotoxin ester derivatives L1–L15 are shown in Scheme 1. These compounds were obtained in three steps which were elucidated in the experimental section. All synthesized compounds were reported and characterized for the first time by melting test, 1H NMR, 13C NMR, mass spectroscopy, and elemental analysis, and results were in accordance with their depicted structures (Table 1).Firstly, all synthesized derivatives L1–L15 were evaluated for their antiproliferation activities against three cancer cell lines [HeLa (human cervical cancer cell), A549 (human lung adenocarcinoma cell), Calu-1 (human lung cancer cell)], and two non-cancer cell lines [L02 (human normal liver cell) and Vero (African green monkey kidney cell)] by MTT assay. The IC50 values defined as the concentrations that cause a 50% loss of cell viability and were shown in Table 2. Results shown in Table 2 demonstrated that lipoyl moiety generally reduced the cytotoxicity of podophyllotoxin toward the non-cancer cells but the effects on cancer cells were slightly weakened. It may be because that the continuous mitotic division of proliferating cancer cells is more sensitive to inhibition of mitosis than non-cancer cells. And this speculation will be validated in the following study. It was heartening that several compounds among them still exhibited the same or even better effect than podophyllotoxin and CA-4 (combretastatin A-4), a typical microtubule disruptor. Generally, their antiproliferation activities against A549 and Calu-1 are better than HeLa cells. To be specific, L5 (IC50 = 4.57 μM), L7 (IC50 = 7.05 μM) and L9 (IC50 = 6.22 μM) showed better effects than podophyllotoxin (IC50 = 8.34 μM) and the effect of L5 even similar to that of CA-4 (IC50 = 5.86 μM) after exposure to HeLa cells. We treated Calu-1 cells with those drugs and found that L3 (IC50 = 9.06 μM), L4 (IC50 = 6.95 μM), L5 (IC50 = 7.28 μM) and L11 (IC50 = 8.13 μM) exhibited better activity than podophyllotoxin (IC50 = 9.47 μM) but their effects are weaker than that of CA-4 (IC50 = 3.65 μM). For A549 cells, L1 (IC50 = 5.89 μM), L3 (IC50 = 5.91 μM), L4 (IC50 = 2.68 μM) and L5 (IC50 = 6.07 μM) are better than podophyllotoxin (IC50 = 6.57 μM). Interestingly, L4 even showed similar effect as CA-4 (IC50 = 2.78 μM). According to the structure–activity relationship, we clearly found that trifluoromethylphenyl, tolyl, methoxyphenyl and chlorophenyl substituted lipoic acid derivatives can significantly improve the antiproliferation activity of podophyllotoxin itself. Among them, trifluoromethylphenyl substituted lipoic acids were better than other derivatives and m-trifluoromethylphenyl substituted lipoic acid was better than the p-trifluoromethylphenyl substituted one. Taken together, we selected L4, the best candidate for cancer treatment in this study, for further investigation.
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HeLa | Calu-1 | A549 | Vero | L02 | |
| a Pod: podophyllotoxin. | |||||
| L1 | 16.2 | 13.6 | 5.89 | 91.2 | 98.6 |
| L2 | 17.3 | 11.2 | 15.0 | >100 | 92.2 |
| L3 | 9.61 | 9.06 | 5.91 | 93.8 | 95.8 |
| L4 | 9.01 | 6.95 | 2.68 | >100 | >100 |
| L5 | 4.57 | 7.28 | 6.07 | >100 | >100 |
| L6 | 18.9 | 19.3 | 12.6 | 99.5 | >100 |
| L7 | 7.05 | 12.4 | 10.6 | 93.2 | >100 |
| L8 | 15.5 | 16.9 | 8.47 | >100 | >100 |
| L9 | 6.22 | 10.4 | 8.82 | 91.6 | >100 |
| L10 | 21.3 | 16.9 | 28.4 | >100 | >100 |
| L11 | 8.36 | 8.13 | 9.38 | >100 | 93.3 |
| L12 | 25.7 | 26.4 | 46.5 | 94.5 | 90.7 |
| L13 | 32.8 | 23.3 | 20.5 | 99.8 | >100 |
| L14 | 27.5 | 11.5 | 16.8 | >100 | >100 |
| L15 | 26.8 | 18.2 | 14.7 | >100 | >100 |
| Podophyllotoxin | 8.34 | 9.47 | 6.57 | 1.04 | 3.16 |
| CA-4 | 5.86 | 3.65 | 2.78 | 2.56 | 4.23 |
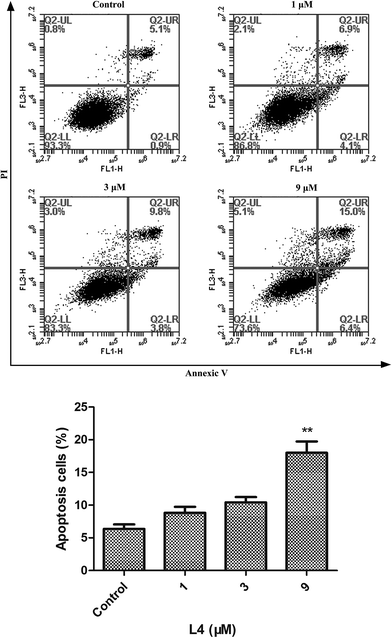
Subsequently, we investigated the effect of L4 on cell apoptosis by flow cytometry. A549 cells were seeded in 6-well-plate for 12 h and then treated with L4 of different concentrations (0, 1, 3, and 9 μM) for 24 h. As shown in Fig. 2, apoptosis was not obvious when treated cells with L4 at low concentrations. And even at the highest concentration (9 μM, three times as much as its IC50 value), it only lead to 21.4% cells apoptosis. Therefore, the effect of L4 on cell apoptosis is not very significant.
To determine the effect of L4 on cell cycle distribution, we treated A549 cells with different doses (0, 0.4, 0.8, and 1.6 μM) of the drug for 8 h and detect cells by flow cytometry. Results were shown in Fig. 3(A) and they demonstrated that L4 can cause cell cycle arrest in G2/M phase remarkably in a dose-dependent manner. Meanwhile, we also designed a time-dependent assay to analysis the effect of L4 on cell cycle distribution. As revealed by Fig. 3(B), the percentage of the cells that arrested in G2/M phase significantly increased with extended exposure time. Specifically, 68.19% cells were arrested in G2/M phase when cells were treated with 0.8 μM L4 for 16 h. Both dose- and time-dependent cell cycle distribution assay results demonstrated that L4 can lead to cell cycle arrest in G2/M potently.
To observe the phenotypic effect of L4 on cellular cytoskeletal network of tubulin, A549 cells were immunostained and analyzed under a confocal microscope. As shown in Fig. 4, L4 inhibited tubulin polymerization and formed polymorphonuclear cells when compared with the control group. The effect of L4 (1.5 μM) on cellular cytoskeletal network of tubulin was similar to that of colchicine (2 μM), but contrary to taxol (2 μM) treated group.
 | ||
| Fig. 4 Effect of L4 on the tubulin network of A549 cells. Microtubules tagged with rhodamine (red) and nuclei tagged with DAPI (blue) were observed under a confocal microscope. | ||
In order to further investigate the effect of L4 on microtubule organization, we did an in vitro microtubule assembly assay. As shown in Fig. 5, A549 cells were treated with L4 (1.5 μM), colchicine (2 μM) and taxol (2 μM) for 16 h, respectively. After the comparison, we found that L4 reduced the expression of polymerized tubulin and the effect was similar to that of colchicine. By contrast, taxol significantly increased the expression of polymerized tubulin. The results suggested that L4 can inhibit tubulin polymerization and the effect was similar to that of colchicine.
Experimental
General information and materials
All chemicals (reagent grade) used were purchased from Nanjing Chemical Reagent Co. Ltd. (China). All the 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 300 or DRX 500 spectrometer in CDCl3. Chemical shifts (δ) for 1H NMR spectra were reported in ppm (δ). ESI mass spectra were obtained on a Mariner Biospectrometry Workstation (ESI-TOF) mass spectrometer. Melting points (uncorrected) were measured on a XT4 MP Apparatus (Taike Corp., Beijing, China). Elemental analyses were performed on a CHN–O-rapid instrument and were within 0.4% of the theoretical values. TLC was carried out on the glass-backed silica gel sheets (silica gel 60 Å GF254) and visualized in UV light (254 nm).Anti-tubulin (#AT819), Cy3-labeled goat anti-mouse IgG (H + L) (#A0521) were purchased from Cytoskeleton, Inc. β-Tubulin antibody (#2146) was purchased from Cell Signaling Technology (Beverly, MA). Goat anti-mouse IgG (H + L) was purchased from Invitrogen Trading (Shanghai) Co., Ltd (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) were purchased from Beyotime Institute of Biotechnology (Haimen, China). AnnexcinV-FITC cell apoptosis assay kit (#BA11100) was purchased from BIO-BOX (Nanjing, China). RNase A (#EN0531) was purchased from Thermo Scientific, Fermentas (USA).
General procedure for preparation of 6,8-dimercaptooctanoic acid
0.5 mol α-lipoic acid was suspended in water (3000 mL), and 0.5 mol NaHCO3 was added. The mixture was stirred to produce a clear solution. The resulting pale yellow solution was cooled in an ice bath, and 1 mol solid NaBH4 was added, with stirring, in small portions. After that, the solution was stirred in an ice bath for another 1 h and then at room temperature for 12 h. The cloudy solution was cooled in an ice bath, and the pH was adjusted to 1 by slow addition of concentrated hydrochloric acid. A vigorous evolution of hydrogen occurred as the excess NaBH4 decomposed, and an oily liquid was seen to separate. The mixture was extracted with chloroform for three times. The combined chloroform extracts were dried over MgSO4, filtered and the solvent evaporated under reduced pressure at room temperature. The remaining oil was further dried under vacuum to remove the residual solvent (Scheme 1).General procedure for preparation of compounds A1–A15
6,8-Dimercaptooctanoic acid (0.03 mol) was dissolved in dichloromethane under a nitrogen atmosphere. To this solution, the appropriate aldehyde (0.03 mol) was added. The mixture was stirred at room temperature for 1 h and cooled to −25 °C. Boron trifluoride etherate (0.03 mol) was added, and the reaction was allowed to warm to room temperature. After evaporation of the solvent, the products were purified by crystallization with ethyl acetate: petroleum ether (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the solvent (Scheme 1).
2) as the solvent (Scheme 1).
General procedure for preparation of compounds L1–L15
Lipoic acid derivatives A1–A15 (0.015 mol), podophyllotoxin (0.01 mol), 4-dimethyaminopyridine (DMAP) (0.001 mol) and N,N′-dicyclohexylcarbodiimide (DCC) (0.02 mol) were dissolved in dichloromethane (30 mL) and stirred for 12 h at room temperature. Adding proper amount of silica gel and condensing solvent by vacuum concentration. Then, collecting target compounds by column chromatography (v(acetone)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (dichloromethane) = 1
(dichloromethane) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Chemical structures of the target compounds (L1–L15) were shown in Table 1.
50). Chemical structures of the target compounds (L1–L15) were shown in Table 1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.14 (t, J = 3.4 Hz, 1H, Ar-CH–S2), 4.58 (d, J = 4.1 Hz, 1H, CH-Ar), 4.34 (dd, J = 9.2, 6.8 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.07–2.75 (m, 5H, O
O), 5.14 (t, J = 3.4 Hz, 1H, Ar-CH–S2), 4.58 (d, J = 4.1 Hz, 1H, CH-Ar), 4.34 (dd, J = 9.2, 6.8 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.07–2.75 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.46–2.36 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.46–2.36 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.17 (d, J = 11.3 Hz, 1H, S–CH2–CH2), 1.71–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.80 (12-C), 173.58 (14-C), 152.57 (3′,5′-C), 148.05 (7-C), 147.53 (6-C), 138.72 (1′′-C), 137.09 (4′-C), 134.82 (1′-C), 132.29 (9-C), 128.71 (2′′,6′′-C), 128.48 (10-C), 128.39 (4′′-C) 127.66 (3′′,5′′-C), 109.65 (8-C), 108.06 (5-C), 106.93 (2′,6′-C), 101.56 (13-C), 73.45 (4-C), 71.28 (11-C), 60.68 (4′-OCH3), 56.10 (3′,5′-OCH3), 52.35 (22-C), 45.95 (2-C), 45.44 (1-C), 43.67 (19-C), 38.67 (3-C), 35.81 (15-C), 34.06 (18-C), 32.70 (20-C), 32.36 (16-C), 25.60 (17-C), 24.76 (21-C). ESI-TOF, calcd for C37H40O9S2 ([M + Na]+) 715.2114, found 715.1162. Anal. calcd for C37H40O9S2: C, 64.14; H, 5.82; O, 20.78; S, 9.26%. Found: C, 63.28; H, 6.54; O, 21.02; S, 9.05%.
O), 2.17 (d, J = 11.3 Hz, 1H, S–CH2–CH2), 1.71–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.80 (12-C), 173.58 (14-C), 152.57 (3′,5′-C), 148.05 (7-C), 147.53 (6-C), 138.72 (1′′-C), 137.09 (4′-C), 134.82 (1′-C), 132.29 (9-C), 128.71 (2′′,6′′-C), 128.48 (10-C), 128.39 (4′′-C) 127.66 (3′′,5′′-C), 109.65 (8-C), 108.06 (5-C), 106.93 (2′,6′-C), 101.56 (13-C), 73.45 (4-C), 71.28 (11-C), 60.68 (4′-OCH3), 56.10 (3′,5′-OCH3), 52.35 (22-C), 45.95 (2-C), 45.44 (1-C), 43.67 (19-C), 38.67 (3-C), 35.81 (15-C), 34.06 (18-C), 32.70 (20-C), 32.36 (16-C), 25.60 (17-C), 24.76 (21-C). ESI-TOF, calcd for C37H40O9S2 ([M + Na]+) 715.2114, found 715.1162. Anal. calcd for C37H40O9S2: C, 64.14; H, 5.82; O, 20.78; S, 9.26%. Found: C, 63.28; H, 6.54; O, 21.02; S, 9.05%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.30 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.40–4.30 (m, 1H, CH–CH2–O), 4.17 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.10–2.72 (m, 5H, O
O), 5.30 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.40–4.30 (m, 1H, CH–CH2–O), 4.17 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.10–2.72 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (s, 3H, –CH3), 2.46–2.37 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (s, 3H, –CH3), 2.46–2.37 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.18 (dd, J = 14.7, 2.4 Hz, 1H, S–CH2–CH2), 1.76–1.46 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.86 (12-C), 173.59 (14-C), 152.64 (3′,5′-C), 148.12 (7-C), 147.59 (6-C), 137.25 (1′′-C), 136.80 (4′-C), 135.04 (2′′-C), 134.83 (1′-C), 132.35 (9-C), 130.50 (3′′-C), 128.38 (6′′-C), 128.19 (10-C), 127.80 (4′′-C), 126.64 (5′′-C), 109.72 (8-C), 108.20 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.52 (4-C), 71.32 (11-C), 60.72 (4′-OCH3), 56.18 (3′,5′-OCH3), 49.05 (22-C), 46.16 (2-C), 45.54 (1-C), 43.74 (19-C), 38.73 (3-C), 35.89 (15-C), 34.12 (18-C), 32.89 (20-C), 32.59 (16-C), 25.67 (17-C), 24.81 (21-C), 19.08 (2′′-CH3). ESI-TOF, calcd for C38H42O9S2 ([M + Na]+) 729.1563, found 729.1212. Anal. calcd for C38H42O9S2: C, 64.57; H, 5.99; O, 20.37; S, 9.07%. Found: C, 63.83; H, 6.29; O, 20.77; S, 8.96%.
O), 2.18 (dd, J = 14.7, 2.4 Hz, 1H, S–CH2–CH2), 1.76–1.46 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.86 (12-C), 173.59 (14-C), 152.64 (3′,5′-C), 148.12 (7-C), 147.59 (6-C), 137.25 (1′′-C), 136.80 (4′-C), 135.04 (2′′-C), 134.83 (1′-C), 132.35 (9-C), 130.50 (3′′-C), 128.38 (6′′-C), 128.19 (10-C), 127.80 (4′′-C), 126.64 (5′′-C), 109.72 (8-C), 108.20 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.52 (4-C), 71.32 (11-C), 60.72 (4′-OCH3), 56.18 (3′,5′-OCH3), 49.05 (22-C), 46.16 (2-C), 45.54 (1-C), 43.74 (19-C), 38.73 (3-C), 35.89 (15-C), 34.12 (18-C), 32.89 (20-C), 32.59 (16-C), 25.67 (17-C), 24.81 (21-C), 19.08 (2′′-CH3). ESI-TOF, calcd for C38H42O9S2 ([M + Na]+) 729.1563, found 729.1212. Anal. calcd for C38H42O9S2: C, 64.57; H, 5.99; O, 20.37; S, 9.07%. Found: C, 63.83; H, 6.29; O, 20.77; S, 8.96%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.12 (s, 1H, Ar-CH–S2), 4.59 (s, 1H, CH-Ar), 4.35 (s, 1H, CH–CH2–O), 4.18 (t, J = 9.1 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 2.88 (dd, J = 32.4, 16.7 Hz, 5H, O
O), 5.12 (s, 1H, Ar-CH–S2), 4.59 (s, 1H, CH-Ar), 4.35 (s, 1H, CH–CH2–O), 4.18 (t, J = 9.1 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 2.88 (dd, J = 32.4, 16.7 Hz, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.36 (d, J = 27.9 Hz, 5H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.36 (d, J = 27.9 Hz, 5H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –CH3), 2.16 (d, J = 12.6 Hz, 1H, S–CH2–CH2), 1.84–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.78 (12-C), 173.56 (14-C), 152.62 (3′,5′-C), 148.08 (7-C), 147.56 (6-C), 138.20 (4′′-C), 137.21 (4′-C), 135.83 (1′-C), 134.87 (1′′-C), 132.35 (9-C), 129.37 (3′′,5′′-C), 128.43 (2′′,6′′-C), 127.53 (10-C), 109.67 (8-C), 108.19 (5-C), 106.95 (2′,6′-C), 101.57 (13-C), 73.49 (4-C), 71.28 (11-C), 60.65 (4′-OCH3), 56.13 (3′,5′-OCH3), 52.10 (22-C), 45.96 (2-C), 45.44 (1-C), 43.72 (19-C), 38.70 (3-C), 35.83 (15-C), 34.09 (18-C), 32.74 (20-C), 32.39 (16-C), 25.62 (17-C), 24.80 (21-C), 21.14 (4′′-CH3). ESI-TOF, calcd for C38H42O9S2 ([M + Na]+) 729.1563, found 729.1212. Anal. calcd for C38H42O9S2: C, 64.57; H, 5.99; O, 20.37; S, 9.07%. Found: C, 63.79; H, 6.22; O, 20.99; S, 8.94%.
O, –CH3), 2.16 (d, J = 12.6 Hz, 1H, S–CH2–CH2), 1.84–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.78 (12-C), 173.56 (14-C), 152.62 (3′,5′-C), 148.08 (7-C), 147.56 (6-C), 138.20 (4′′-C), 137.21 (4′-C), 135.83 (1′-C), 134.87 (1′′-C), 132.35 (9-C), 129.37 (3′′,5′′-C), 128.43 (2′′,6′′-C), 127.53 (10-C), 109.67 (8-C), 108.19 (5-C), 106.95 (2′,6′-C), 101.57 (13-C), 73.49 (4-C), 71.28 (11-C), 60.65 (4′-OCH3), 56.13 (3′,5′-OCH3), 52.10 (22-C), 45.96 (2-C), 45.44 (1-C), 43.72 (19-C), 38.70 (3-C), 35.83 (15-C), 34.09 (18-C), 32.74 (20-C), 32.39 (16-C), 25.62 (17-C), 24.80 (21-C), 21.14 (4′′-CH3). ESI-TOF, calcd for C38H42O9S2 ([M + Na]+) 729.1563, found 729.1212. Anal. calcd for C38H42O9S2: C, 64.57; H, 5.99; O, 20.37; S, 9.07%. Found: C, 63.79; H, 6.22; O, 20.99; S, 8.94%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.19 (s, 1H, Ar-CH–S2), 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.35 (dd, J = 9.2, 6.9 Hz, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.07–2.74 (m, 5H, O
O), 5.19 (s, 1H, Ar-CH–S2), 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.35 (dd, J = 9.2, 6.9 Hz, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.07–2.74 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.31 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.31 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.25–2.13 (m, 1H, S–CH2–CH2), 1.98–1.49 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (75 MHz, CDCl3) δ 173.73 (12-C), 173.50 (14-C), 152.52 (3′,5′-C), 148.01 (7-C), 147.48 (6-C), 139.75 (1′′-C) 139.66 (4′-C), 137.06 (1′-C), 134.73 (6′′-C), 132.22 (3′′-C), 131.12 (9-C), 129.18 (5′′-C), 128.20 (10-C), 125.17 (2′′-C), 124.61 (3′′-CF3), 124.57 (4′′-C), 109.59 (8-C), 108.03 (5-C), 106.84 (2′,6′-C), 101.48 (13-C), 73.42 (4-C), 71.22 (11-C), 60.59 (4′-OCH3), 56.03 (3′,5′-OCH3), 49.69 (22-C), 45.91 (2-C), 45.42 (1-C), 43.61 (19-C), 38.63 (3-C), 33.88 (15-C), 32.58 (18-C), 32.41 (20-C), 30.81 (16-C), 26.16 (17-C), 25.51 (21-C). ESI-TOF, calcd for C38H39F3O9S2 ([M + Na]+) 783.1988, found 783.1549. Anal. calcd for C38H39F3O9S2: C, 59.99; H, 5.17; F, 7.49; O, 18.93; S, 8.43%. Found: C, 59.01; H, 5.92; F, 7.44; O, 20.19; S, 8.22%.
O), 2.25–2.13 (m, 1H, S–CH2–CH2), 1.98–1.49 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (75 MHz, CDCl3) δ 173.73 (12-C), 173.50 (14-C), 152.52 (3′,5′-C), 148.01 (7-C), 147.48 (6-C), 139.75 (1′′-C) 139.66 (4′-C), 137.06 (1′-C), 134.73 (6′′-C), 132.22 (3′′-C), 131.12 (9-C), 129.18 (5′′-C), 128.20 (10-C), 125.17 (2′′-C), 124.61 (3′′-CF3), 124.57 (4′′-C), 109.59 (8-C), 108.03 (5-C), 106.84 (2′,6′-C), 101.48 (13-C), 73.42 (4-C), 71.22 (11-C), 60.59 (4′-OCH3), 56.03 (3′,5′-OCH3), 49.69 (22-C), 45.91 (2-C), 45.42 (1-C), 43.61 (19-C), 38.63 (3-C), 33.88 (15-C), 32.58 (18-C), 32.41 (20-C), 30.81 (16-C), 26.16 (17-C), 25.51 (21-C). ESI-TOF, calcd for C38H39F3O9S2 ([M + Na]+) 783.1988, found 783.1549. Anal. calcd for C38H39F3O9S2: C, 59.99; H, 5.17; F, 7.49; O, 18.93; S, 8.43%. Found: C, 59.01; H, 5.92; F, 7.44; O, 20.19; S, 8.22%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.19 (d, J = 2.4 Hz, 1H, Ar-CH–S2), 4.60 (d, J = 4.1 Hz, 1H, CH-Ar), 4.40–4.31 (m, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.07–2.76 (m, 5H, O
O), 5.19 (d, J = 2.4 Hz, 1H, Ar-CH–S2), 4.60 (d, J = 4.1 Hz, 1H, CH-Ar), 4.40–4.31 (m, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.07–2.76 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (dt, J = 12.0, 7.7 Hz, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (dt, J = 12.0, 7.7 Hz, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.20 (d, J = 12.9 Hz, 1H, S–CH2–CH2), 1.65 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.83 (12-C), 173.55 (14-C), 152.65 (3′,5′-C), 148.14 (7-C), 147.60 (6-C), 142.64 (1′′-C), 137.37 (4′-C), 137.18 (1′-C), 134.80 (9-C), 132.38 (4′′-C), 128.32 (2′′,6′′-C), 128.23 (10-C), 125.75 (3′′,5′′-C), 125.67 (4′′-CF3), 109.74 (8-C), 108.20 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.55 (4-C), 71.32 (11-C), 60.73 (4′-OCH3), 56.18 (3′,5′-OCH3), 51.79 (22-C), 46.01 (2-C), 45.56 (1-C), 43.74 (19-C), 38.75 (3-C), 35.81 (15-C), 34.09 (18-C), 32.58 (20-C), 32.33 (16-C), 25.61 (17-C), 24.79 (21-C). ESI-TOF, calcd for C38H39F3O9S2 ([M + Na]+) 783.1988, found 783.1549. Anal. calcd for C38H39F3O9S2: C, 59.99; H, 5.17; F, 7.49; O, 18.93; S, 8.43%. Found: C, 59.04; H, 5.96; F, 7.44; O, 19.12; S, 8.41%.
O), 2.20 (d, J = 12.9 Hz, 1H, S–CH2–CH2), 1.65 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.83 (12-C), 173.55 (14-C), 152.65 (3′,5′-C), 148.14 (7-C), 147.60 (6-C), 142.64 (1′′-C), 137.37 (4′-C), 137.18 (1′-C), 134.80 (9-C), 132.38 (4′′-C), 128.32 (2′′,6′′-C), 128.23 (10-C), 125.75 (3′′,5′′-C), 125.67 (4′′-CF3), 109.74 (8-C), 108.20 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.55 (4-C), 71.32 (11-C), 60.73 (4′-OCH3), 56.18 (3′,5′-OCH3), 51.79 (22-C), 46.01 (2-C), 45.56 (1-C), 43.74 (19-C), 38.75 (3-C), 35.81 (15-C), 34.09 (18-C), 32.58 (20-C), 32.33 (16-C), 25.61 (17-C), 24.79 (21-C). ESI-TOF, calcd for C38H39F3O9S2 ([M + Na]+) 783.1988, found 783.1549. Anal. calcd for C38H39F3O9S2: C, 59.99; H, 5.17; F, 7.49; O, 18.93; S, 8.43%. Found: C, 59.04; H, 5.96; F, 7.44; O, 19.12; S, 8.41%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.69 (s, 1H, Ar-CH–S2), 4.59 (d, J = 4.2 Hz, 1H, CH-Ar), 4.35 (dd, J = 9.2, 6.8 Hz, 1H, CH–CH2–O), 4.18 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.85 (s, 3H, –OCH3), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.13–2.75 (m, 5H, O
O), 5.69 (s, 1H, Ar-CH–S2), 4.59 (d, J = 4.2 Hz, 1H, CH-Ar), 4.35 (dd, J = 9.2, 6.8 Hz, 1H, CH–CH2–O), 4.18 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.85 (s, 3H, –OCH3), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.13–2.75 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.34 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.34 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.17 (d, J = 11.9 Hz, 1H, S–CH2–CH2), 1.78–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.88 (12-C), 173.62 (14-C), 155.41 (2′-C), 152.63 (3′,5′-C), 148.11 (7-C), 147.59 (6-C), 137.16 (4′-C), 134.84 (1′-C), 132.32 (9-C), 129.43 (6′′-C), 129.14 (4′′-C), 128.37 (10-C), 126.86 (1′′-C), 121.05 (5′′-C), 110.80 (3′′-C), 109.70 (8-C), 108.12 (5-C), 106.96 (2′,6′-C), 101.59 (13-C), 73.50 (4-C), 71.33 (11-C), 60.73 (4′-OCH3), 56.15 (3′,5′-OCH3, 2′-OCH3), 55.74 (22-C), 46.07 (2-C), 45.53 (1-C), 44.37 (19-C), 43.73 (3-C), 38.71 (15-C), 35.90 (18-C), 32.90 (20-C), 32.60 (16-C), 25.67 (17-C), 24.82 (21-C). ESI-TOF, calcd for C38H42O10S2 ([M + Na]+) 745.2219, found 745.0016. Anal. calcd for C38H42O10S2: C, 63.14; H, 5.86; O, 22.13; S, 8.87%. Found: C, 62.53; H, 6.18; O, 22.43; S, 8.79%.
O), 2.17 (d, J = 11.9 Hz, 1H, S–CH2–CH2), 1.78–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.88 (12-C), 173.62 (14-C), 155.41 (2′-C), 152.63 (3′,5′-C), 148.11 (7-C), 147.59 (6-C), 137.16 (4′-C), 134.84 (1′-C), 132.32 (9-C), 129.43 (6′′-C), 129.14 (4′′-C), 128.37 (10-C), 126.86 (1′′-C), 121.05 (5′′-C), 110.80 (3′′-C), 109.70 (8-C), 108.12 (5-C), 106.96 (2′,6′-C), 101.59 (13-C), 73.50 (4-C), 71.33 (11-C), 60.73 (4′-OCH3), 56.15 (3′,5′-OCH3, 2′-OCH3), 55.74 (22-C), 46.07 (2-C), 45.53 (1-C), 44.37 (19-C), 43.73 (3-C), 38.71 (15-C), 35.90 (18-C), 32.90 (20-C), 32.60 (16-C), 25.67 (17-C), 24.82 (21-C). ESI-TOF, calcd for C38H42O10S2 ([M + Na]+) 745.2219, found 745.0016. Anal. calcd for C38H42O10S2: C, 63.14; H, 5.86; O, 22.13; S, 8.87%. Found: C, 62.53; H, 6.18; O, 22.43; S, 8.79%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.12 (s, 1H, Ar-CH–S2), 4.57 (d, J = 3.7 Hz, 1H, CH-Ar), 4.35 (t, J = 7.8 Hz, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.88 (s, 3H, –OCH3), 3.85 (s, 3H, –OCH3), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.08–2.73 (m, 5H, O
O), 5.12 (s, 1H, Ar-CH–S2), 4.57 (d, J = 3.7 Hz, 1H, CH-Ar), 4.35 (t, J = 7.8 Hz, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.88 (s, 3H, –OCH3), 3.85 (s, 3H, –OCH3), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.08–2.73 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.43 (dd, J = 11.1, 7.0 Hz, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.43 (dd, J = 11.1, 7.0 Hz, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.17 (d, J = 10.8 Hz, 1H, S–CH2–CH2), 1.77–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.82 (12-C), 173.56 (14-C), 152.62 (3′,5′-C), 149.09 (3′′,4′′-C), 148.09 (7-C), 147.56 (6-C), 137.23 (4′-C), 134.82 (1′-C), 132.34 (9-C), 131.31 (1′′-C), 128.36 (10-C), 120.03 (6′′-C), 111.26 (2′′-C), 110.85 (5′′-C), 109.69 (8-C), 108.19 (5-C), 106.93 (2′,6′-C), 101.57 (13-C), 73.50 (4-C), 71.29 (11-C), 60.69 (4′-OCH3), 56.16 (3′,5′-OCH3), 55.90 (3′′,4′′-OCH3), 52.13 (22-C), 46.06 (2-C), 45.49 (1-C), 43.71 (19-C), 38.71 (3-C), 35.83 (15-C), 34.10 (18-C), 32.69 (20-C), 32.46 (16-C), 25.65 (17-C), 24.79 (21-C). ESI-TOF, calcd for C39H44O11S2 ([M + Na]+) 775.2325, found 775.0075. Anal. calcd for C39H44O11S2: C, 62.22; H, 5.89; O, 23.38; S, 8.52%. Found: C, 61.64; H, 6.02; O, 23.57; S, 8.53%.
O), 2.17 (d, J = 10.8 Hz, 1H, S–CH2–CH2), 1.77–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.82 (12-C), 173.56 (14-C), 152.62 (3′,5′-C), 149.09 (3′′,4′′-C), 148.09 (7-C), 147.56 (6-C), 137.23 (4′-C), 134.82 (1′-C), 132.34 (9-C), 131.31 (1′′-C), 128.36 (10-C), 120.03 (6′′-C), 111.26 (2′′-C), 110.85 (5′′-C), 109.69 (8-C), 108.19 (5-C), 106.93 (2′,6′-C), 101.57 (13-C), 73.50 (4-C), 71.29 (11-C), 60.69 (4′-OCH3), 56.16 (3′,5′-OCH3), 55.90 (3′′,4′′-OCH3), 52.13 (22-C), 46.06 (2-C), 45.49 (1-C), 43.71 (19-C), 38.71 (3-C), 35.83 (15-C), 34.10 (18-C), 32.69 (20-C), 32.46 (16-C), 25.65 (17-C), 24.79 (21-C). ESI-TOF, calcd for C39H44O11S2 ([M + Na]+) 775.2325, found 775.0075. Anal. calcd for C39H44O11S2: C, 62.22; H, 5.89; O, 23.38; S, 8.52%. Found: C, 61.64; H, 6.02; O, 23.57; S, 8.53%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.13 (s, 1H, Ar-CH–S2), 4.58 (d, J = 3.7 Hz, 1H, CH-Ar), 4.40–4.29 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.05–2.74 (m, 6H, O
O), 5.13 (s, 1H, Ar-CH–S2), 4.58 (d, J = 3.7 Hz, 1H, CH-Ar), 4.40–4.29 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.05–2.74 (m, 6H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S, CH–(CH3)2), 2.41 (dd, J = 10.8, 7.0 Hz, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S, CH–(CH3)2), 2.41 (dd, J = 10.8, 7.0 Hz, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.16 (d, J = 13.5 Hz, 1H, S–CH2–CH2), 1.78–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2), 1.22 (s, 3H, –CH3), 1.20 (s, 3H, –CH3). 13C NMR (126 MHz, CDCl3) δ 173.85 (12-C), 173.59 (14-C), 152.64 (3′,5′-C), 149.17 (4′′-C), 148.11 (7-C), 147.59 (6-C), 137.22 (4′-C), 136.07 (1′-C), 134.84 (9-C), 132.35 (1′′-C), 128.39 (2′′,6′′-C), 127.59 (10-C), 126.82 (3′′,5′′-C), 109.70 (8-C), 108.17 (5-C), 106.97 (2′,6′-C), 101.59 (13-C), 73.51 (4-C), 71.32 (11-C), 60.71 (4′-OCH3), 56.16 (3′,5′-OCH3), 52.14 (22-C), 46.01 (2-C), 45.52 (1-C), 43.74 (19-C), 38.72 (3-C), 35.86 (15-C), 34.13 (18-C), 33.84 (4′′-CH–(CH3)), 32.79 (20-C), 32.45 (16-C), 25.66 (17-C), 24.81 (21-C), 23.88 (4′′-CH–(CH3)). ESI-TOF, calcd for C40H46O9S2 ([M + Na]+) 757.2583, found 757.1351. Anal. calcd for C40H46O9S2: C, 65.37; H, 6.31; O, 19.59; S, 8.73%. Found: C, 64.73; H, 6.59; O, 20.09; S, 8.63%.
O), 2.16 (d, J = 13.5 Hz, 1H, S–CH2–CH2), 1.78–1.47 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2), 1.22 (s, 3H, –CH3), 1.20 (s, 3H, –CH3). 13C NMR (126 MHz, CDCl3) δ 173.85 (12-C), 173.59 (14-C), 152.64 (3′,5′-C), 149.17 (4′′-C), 148.11 (7-C), 147.59 (6-C), 137.22 (4′-C), 136.07 (1′-C), 134.84 (9-C), 132.35 (1′′-C), 128.39 (2′′,6′′-C), 127.59 (10-C), 126.82 (3′′,5′′-C), 109.70 (8-C), 108.17 (5-C), 106.97 (2′,6′-C), 101.59 (13-C), 73.51 (4-C), 71.32 (11-C), 60.71 (4′-OCH3), 56.16 (3′,5′-OCH3), 52.14 (22-C), 46.01 (2-C), 45.52 (1-C), 43.74 (19-C), 38.72 (3-C), 35.86 (15-C), 34.13 (18-C), 33.84 (4′′-CH–(CH3)), 32.79 (20-C), 32.45 (16-C), 25.66 (17-C), 24.81 (21-C), 23.88 (4′′-CH–(CH3)). ESI-TOF, calcd for C40H46O9S2 ([M + Na]+) 757.2583, found 757.1351. Anal. calcd for C40H46O9S2: C, 65.37; H, 6.31; O, 19.59; S, 8.73%. Found: C, 64.73; H, 6.59; O, 20.09; S, 8.63%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.62 (s, 1H, Ar-CH–S2), 4.59 (d, J = 3.4 Hz, 1H, CH-Ar), 4.42–4.30 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.16–2.72 (m, 5H, O
O), 5.62 (s, 1H, Ar-CH–S2), 4.59 (d, J = 3.4 Hz, 1H, CH-Ar), 4.42–4.30 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.6 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.16–2.72 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.41 (d, J = 5.5 Hz, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.41 (d, J = 5.5 Hz, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.19 (d, J = 12.5 Hz, 1H, S–CH2–CH2), 2.00–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.81 (12-C), 173.58 (14-C), 152.63 (3′,5′-C), 148.11 (7-C), 147.58 (6-C), 137.24 (1′′-C), 136.22 (4′-C), 134.82 (1′-C), 132.35 (9-C), 129.62 (2′′,6′′-C), 129.46 (5′′-C), 128.38 (10-C), 127.48 (3′′,4′′-C), 109.70 (8-C), 108.20 (5-C), 106.94 (2′,6′-C), 101.58 (13-C), 73.52 (4-C), 71.31 (11-C), 60.70 (4′-OCH3), 56.16 (3′,5′-OCH3), 49.76 (22-C), 48.41 (s), 46.09 (2-C), 45.53 (1-C), 43.73 (19-C), 38.71 (3-C), 35.81 (15-C), 34.08 (18-C), 32.71 (20-C), 32.49 (16-C), 30.92 (s), 25.60 (17-C), 24.78 (21-C). ESI-TOF, calcd for C37H39ClO9S2 ([M + Na]+) 759.1724, found 759.1003. Anal. calcd for C37H39ClO9S2: C, 61.10; H, 5.41; Cl, 4.87; O, 19.80; S, 8.82%. Found: C, 59.76; H, 5.88; Cl, 4.90; O, 20.32; S, 8.80%.
O), 2.19 (d, J = 12.5 Hz, 1H, S–CH2–CH2), 2.00–1.45 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.81 (12-C), 173.58 (14-C), 152.63 (3′,5′-C), 148.11 (7-C), 147.58 (6-C), 137.24 (1′′-C), 136.22 (4′-C), 134.82 (1′-C), 132.35 (9-C), 129.62 (2′′,6′′-C), 129.46 (5′′-C), 128.38 (10-C), 127.48 (3′′,4′′-C), 109.70 (8-C), 108.20 (5-C), 106.94 (2′,6′-C), 101.58 (13-C), 73.52 (4-C), 71.31 (11-C), 60.70 (4′-OCH3), 56.16 (3′,5′-OCH3), 49.76 (22-C), 48.41 (s), 46.09 (2-C), 45.53 (1-C), 43.73 (19-C), 38.71 (3-C), 35.81 (15-C), 34.08 (18-C), 32.71 (20-C), 32.49 (16-C), 30.92 (s), 25.60 (17-C), 24.78 (21-C). ESI-TOF, calcd for C37H39ClO9S2 ([M + Na]+) 759.1724, found 759.1003. Anal. calcd for C37H39ClO9S2: C, 61.10; H, 5.41; Cl, 4.87; O, 19.80; S, 8.82%. Found: C, 59.76; H, 5.88; Cl, 4.90; O, 20.32; S, 8.80%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.10 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.34 (dd, J = 8.9, 7.0 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.04–2.78 (m, 5H, O
O), 5.10 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.34 (dd, J = 8.9, 7.0 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.04–2.78 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.39 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.39 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.16 (d, J = 13.3 Hz, 1H, S–CH2–CH2), 1.84–1.44 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.83 (12-C), 173.59 (14-C), 152.61 (3′,5′-C), 148.11 (7-C), 147.57 (6-C), 137.30 (4′-C), 137.15 (1′-C), 134.83 (9-C), 134.12 (1′′-C), 132.33 (4′′-C), 129.13 (2′′,6′′-C), 128.92 (3′′,5′′-C), 128.33 (10-C), 109.71 (8-C), 108.13 (5-C), 106.96 (2′,6′-C), 101.60 (13-C), 73.52 (4-C), 71.33 (11-C), 60.72 (4′-OCH3), 56.15 (3′,5′-OCH3), 51.51 (22-C), 45.98 (2-C), 45.51 (1-C), 43.71 (19-C), 38.73 (3-C), 35.80 (15-C), 34.09 (18-C), 32.58 (20-C), 32.34 (16-C), 25.60 (17-C), 24.78 (21-C). ESI-TOF, calcd for C37H39ClO9S2 ([M + Na]+) 759.1724, found 759.1003. Anal. calcd for C37H39ClO9S2: C, 61.10; H, 5.41; Cl, 4.87; O, 19.80; S, 8.82%. Found: C, 60.43; H, 5.82; Cl, 4.95; O, 20.52; S, 8.81%.
O), 2.16 (d, J = 13.3 Hz, 1H, S–CH2–CH2), 1.84–1.44 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.83 (12-C), 173.59 (14-C), 152.61 (3′,5′-C), 148.11 (7-C), 147.57 (6-C), 137.30 (4′-C), 137.15 (1′-C), 134.83 (9-C), 134.12 (1′′-C), 132.33 (4′′-C), 129.13 (2′′,6′′-C), 128.92 (3′′,5′′-C), 128.33 (10-C), 109.71 (8-C), 108.13 (5-C), 106.96 (2′,6′-C), 101.60 (13-C), 73.52 (4-C), 71.33 (11-C), 60.72 (4′-OCH3), 56.15 (3′,5′-OCH3), 51.51 (22-C), 45.98 (2-C), 45.51 (1-C), 43.71 (19-C), 38.73 (3-C), 35.80 (15-C), 34.09 (18-C), 32.58 (20-C), 32.34 (16-C), 25.60 (17-C), 24.78 (21-C). ESI-TOF, calcd for C37H39ClO9S2 ([M + Na]+) 759.1724, found 759.1003. Anal. calcd for C37H39ClO9S2: C, 61.10; H, 5.41; Cl, 4.87; O, 19.80; S, 8.82%. Found: C, 60.43; H, 5.82; Cl, 4.95; O, 20.52; S, 8.81%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.63 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.34 (dd, J = 8.9, 7.0 Hz, 1H, CH–CH2–O), 4.17 (td, J = 9.9, 2.0 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.17–2.70 (m, 5H, O
O), 5.63 (s, 1H, Ar-CH–S2), 4.58 (d, J = 4.0 Hz, 1H, CH-Ar), 4.34 (dd, J = 8.9, 7.0 Hz, 1H, CH–CH2–O), 4.17 (td, J = 9.9, 2.0 Hz, 1H, CH–CH2–O), 3.79 (s, 3H, 4′-OCH3), 3.74 (s, 6H, 3′,5′-OCH3), 3.17–2.70 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.36 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.36 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.18 (d, J = 12.3 Hz, 1H, S–CH2–CH2), 1.79–1.43 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.84 (12-C), 173.60 (14-C), 152.62 (3′,5′-C), 148.11 (7-C), 147.58 (6-C), 138.48 (1′′-C), 137.18 (4′-C), 134.82 (1′-C), 133.23 (3′′-C), 132.33 (9-C), 130.87 (2′′-C), 130.24 (4′′-C), 128.33 (10-C), 127.83 (5′′,6′′-C), 109.72 (8-C), 108.14 (5-C), 106.95 (2′,6′-C), 101.60 (13-C), 73.53 (4-C), 71.34 (11-C), 60.74 (4′-OCH3), 56.17 (3′,5′-OCH3), 49.13 (22-C), 46.18 (2-C), 45.54 (1-C), 43.72 (19-C), 38.72 (3-C), 35.78 (15-C), 34.07 (18-C), 32.66 (20-C), 32.52 (16-C), 25.59 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H38Cl2O9S2 ([M + Na]+) 783.1334, found 783.1095. Anal. calcd for C37H38Cl2O9S2: C, 58.34; H, 5.03; Cl, 9.31; O, 18.90; S, 8.42%. Found: C, 57.65; H, 5.75; Cl, 9.42; O, 20.04; S, 8.40%.
O), 2.18 (d, J = 12.3 Hz, 1H, S–CH2–CH2), 1.79–1.43 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.84 (12-C), 173.60 (14-C), 152.62 (3′,5′-C), 148.11 (7-C), 147.58 (6-C), 138.48 (1′′-C), 137.18 (4′-C), 134.82 (1′-C), 133.23 (3′′-C), 132.33 (9-C), 130.87 (2′′-C), 130.24 (4′′-C), 128.33 (10-C), 127.83 (5′′,6′′-C), 109.72 (8-C), 108.14 (5-C), 106.95 (2′,6′-C), 101.60 (13-C), 73.53 (4-C), 71.34 (11-C), 60.74 (4′-OCH3), 56.17 (3′,5′-OCH3), 49.13 (22-C), 46.18 (2-C), 45.54 (1-C), 43.72 (19-C), 38.72 (3-C), 35.78 (15-C), 34.07 (18-C), 32.66 (20-C), 32.52 (16-C), 25.59 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H38Cl2O9S2 ([M + Na]+) 783.1334, found 783.1095. Anal. calcd for C37H38Cl2O9S2: C, 58.34; H, 5.03; Cl, 9.31; O, 18.90; S, 8.42%. Found: C, 57.65; H, 5.75; Cl, 9.42; O, 20.04; S, 8.40%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.56 (s, 1H, Ar-CH–S2), 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.36 (dd, J = 9.3, 6.8 Hz, 1H, CH–CH2–O), 4.19 (t, J = 9.5 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.15–2.80 (m, 5H, O
O), 5.56 (s, 1H, Ar-CH–S2), 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.36 (dd, J = 9.3, 6.8 Hz, 1H, CH–CH2–O), 4.19 (t, J = 9.5 Hz, 1H, CH–CH2–O), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.15–2.80 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.49–2.37 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.49–2.37 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.20 (d, J = 11.8 Hz, 1H, S–CH2–CH2), 1.77–1.48 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.81 (12-C), 173.56 (14-C), 152.65 (3′,5′-C), 148.12 (7-C), 147.59 (6-C), 134.92 (1′′-C), 134.80 (4′-C), 134.57 (1′-C), 133.17 (2′′-C), 132.37 (9-C), 131.23 (4′′-C), 130.54 (6′′-C), 129.40 (3′′-C), 128.34 (10-C), 127.84 (5′′-C), 109.73 (8-C), 108.21 (5-C), 106.94 (2′,6′-C), 101.59 (13-C), 73.55 (4-C), 71.32 (11-C), 60.72 (4′-OCH3), 56.18 (3′,5′-OCH3), 47.74 (22-C), 46.10 (2-C), 45.55 (1-C), 43.74 (19-C), 38.73 (3-C), 35.78 (15-C), 34.07 (18-C), 32.59 (20-C), 32.44 (16-C), 25.59 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H38Cl2O9S2 ([M + Na]+) 783.1334, found 783.1095. Anal. calcd for C37H38Cl2O9S2: C, 58.34; H, 5.03; Cl, 9.31; O, 18.90; S, 8.42%. Found: C, 57.55; H, 5.63; Cl, 9.46; O, 19.56; S, 8.40%.
O), 2.20 (d, J = 11.8 Hz, 1H, S–CH2–CH2), 1.77–1.48 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.81 (12-C), 173.56 (14-C), 152.65 (3′,5′-C), 148.12 (7-C), 147.59 (6-C), 134.92 (1′′-C), 134.80 (4′-C), 134.57 (1′-C), 133.17 (2′′-C), 132.37 (9-C), 131.23 (4′′-C), 130.54 (6′′-C), 129.40 (3′′-C), 128.34 (10-C), 127.84 (5′′-C), 109.73 (8-C), 108.21 (5-C), 106.94 (2′,6′-C), 101.59 (13-C), 73.55 (4-C), 71.32 (11-C), 60.72 (4′-OCH3), 56.18 (3′,5′-OCH3), 47.74 (22-C), 46.10 (2-C), 45.55 (1-C), 43.74 (19-C), 38.73 (3-C), 35.78 (15-C), 34.07 (18-C), 32.59 (20-C), 32.44 (16-C), 25.59 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H38Cl2O9S2 ([M + Na]+) 783.1334, found 783.1095. Anal. calcd for C37H38Cl2O9S2: C, 58.34; H, 5.03; Cl, 9.31; O, 18.90; S, 8.42%. Found: C, 57.55; H, 5.63; Cl, 9.46; O, 19.56; S, 8.40%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.09 (s, 1H, Ar-CH–S2), 4.59 (d, J = 3.7 Hz, 1H, CH-Ar), 4.40–4.29 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.08–2.72 (m, 5H, O
O), 5.09 (s, 1H, Ar-CH–S2), 4.59 (d, J = 3.7 Hz, 1H, CH-Ar), 4.40–4.29 (m, 1H, CH–CH2–O), 4.18 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.80 (s, 3H, 4′-OCH3), 3.75 (s, 6H, 3′,5′-OCH3), 3.08–2.72 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (dd, J = 9.7, 6.2 Hz, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.42 (dd, J = 9.7, 6.2 Hz, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.17 (d, J = 13.1 Hz, 1H, S–CH2–CH2), 1.60 (ddd, J = 26.9, 23.9, 16.1 Hz, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.82 (12-C), 173.60 (14-C), 152.61 (3′,5′-C), 148.10 (7-C), 147.57 (6-C), 140.94 (1′′-C), 137.15 (4′-C), 134.85 (1′-C), 132.32 (9-C), 131.55 (2′′-C), 130.84 (4′′-C), 130.30 (5′′-C), 128.34 (10-C), 126.45 (6′′-C), 122.55 (3′′-C), 109.69 (8-C), 108.12 (5-C), 106.96 (2′,6′-C), 101.60 (13-C), 73.51 (4-C), 71.32 (11-C), 60.70 (4′-OCH3), 56.14 (3′,5′-OCH3), 51.58 (22-C), 45.98 (2-C), 45.49 (1-C), 43.70 (19-C), 38.72 (3-C), 35.79 (15-C), 34.09 (18-C), 32.59 (20-C), 32.32 (16-C), 25.61 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H39BrO9S2 ([M + Na]+) 793.1219, found 793.0043. Anal. calcd for C37H39BrO9S2: C, 57.58; H, 5.09; Br, 10.35; O, 18.66; S, 8.31%. Found: C, 56.63; H, 5.78; Br, 10.42; O, 19.02; S, 8.29%.
O), 2.17 (d, J = 13.1 Hz, 1H, S–CH2–CH2), 1.60 (ddd, J = 26.9, 23.9, 16.1 Hz, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.82 (12-C), 173.60 (14-C), 152.61 (3′,5′-C), 148.10 (7-C), 147.57 (6-C), 140.94 (1′′-C), 137.15 (4′-C), 134.85 (1′-C), 132.32 (9-C), 131.55 (2′′-C), 130.84 (4′′-C), 130.30 (5′′-C), 128.34 (10-C), 126.45 (6′′-C), 122.55 (3′′-C), 109.69 (8-C), 108.12 (5-C), 106.96 (2′,6′-C), 101.60 (13-C), 73.51 (4-C), 71.32 (11-C), 60.70 (4′-OCH3), 56.14 (3′,5′-OCH3), 51.58 (22-C), 45.98 (2-C), 45.49 (1-C), 43.70 (19-C), 38.72 (3-C), 35.79 (15-C), 34.09 (18-C), 32.59 (20-C), 32.32 (16-C), 25.61 (17-C), 24.77 (21-C). ESI-TOF, calcd for C37H39BrO9S2 ([M + Na]+) 793.1219, found 793.0043. Anal. calcd for C37H39BrO9S2: C, 57.58; H, 5.09; Br, 10.35; O, 18.66; S, 8.31%. Found: C, 56.63; H, 5.78; Br, 10.42; O, 19.02; S, 8.29%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 5.14 (s, 1H, –OH), 5.11 (s, 1H, Ar-CH–S2) 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.41–4.32 (m, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.90 (s, 3H, –OCH3), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.07–2.74 (m, 5H, O
O), 5.14 (s, 1H, –OH), 5.11 (s, 1H, Ar-CH–S2) 4.60 (d, J = 4.2 Hz, 1H, CH-Ar), 4.41–4.32 (m, 1H, CH–CH2–O), 4.19 (t, J = 9.7 Hz, 1H, CH–CH2–O), 3.90 (s, 3H, –OCH3), 3.81 (s, 3H, 4′-OCH3), 3.76 (s, 6H, 3′,5′-OCH3), 3.07–2.74 (m, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.36 (m, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.48–2.36 (m, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.18 (s, 1H, S–CH2–CH2), 1.81–1.48 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.89 (12-C), 173.63 (14-C), 152.64 (3′,5′-C), 151.22 (4′′-C), 148.13 (7-C), 147.59 (6-C), 146.66 (3′′-C), 137.56 (4′-C), 137.19 (1′-C), 134.83 (1′′-C), 132.33 (9-C), 130.72 (6′′-C), 128.32 (10-C), 120.09 (2′′-C), 114.48 (5′′-C), 109.72 (8-C), 108.15 (5-C), 106.97 (2′,6′-C), 101.60 (13-C), 73.53 (4-C), 71.35 (11-C), 60.74 (4′-OCH3), 56.18 (3′,5′-OCH3), 55.96 (4′′-OCH3), 52.24 (22-C), 46.07 (2-C), 45.57 (1-C), 43.73 (19-C), 38.74 (3-C), 35.81 (15-C), 34.12 (18-C), 32.50 (16-C), 25.55 (17-C), 24.89 (21-C). ESI-TOF, calcd for C38H42O11S2 ([M + Na]+) 761.2169, found 761.1126. Anal. calcd for C38H42O11S2: C, 61.77; H, 5.73; O, 23.82; S, 8.68%. Found: C, 60.97; H, 6.03; O, 24.16; S, 8.66%.
O), 2.18 (s, 1H, S–CH2–CH2), 1.81–1.48 (m, 7H, S–CH2–CH2, S–CH–CH2–CH2–CH2). 13C NMR (126 MHz, CDCl3) δ 173.89 (12-C), 173.63 (14-C), 152.64 (3′,5′-C), 151.22 (4′′-C), 148.13 (7-C), 147.59 (6-C), 146.66 (3′′-C), 137.56 (4′-C), 137.19 (1′-C), 134.83 (1′′-C), 132.33 (9-C), 130.72 (6′′-C), 128.32 (10-C), 120.09 (2′′-C), 114.48 (5′′-C), 109.72 (8-C), 108.15 (5-C), 106.97 (2′,6′-C), 101.60 (13-C), 73.53 (4-C), 71.35 (11-C), 60.74 (4′-OCH3), 56.18 (3′,5′-OCH3), 55.96 (4′′-OCH3), 52.24 (22-C), 46.07 (2-C), 45.57 (1-C), 43.73 (19-C), 38.74 (3-C), 35.81 (15-C), 34.12 (18-C), 32.50 (16-C), 25.55 (17-C), 24.89 (21-C). ESI-TOF, calcd for C38H42O11S2 ([M + Na]+) 761.2169, found 761.1126. Anal. calcd for C38H42O11S2: C, 61.77; H, 5.73; O, 23.82; S, 8.68%. Found: C, 60.97; H, 6.03; O, 24.16; S, 8.66%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 4.57 (d, J = 3.1 Hz, 1H, CH-Ar), 4.34 (t, J = 7.7 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.6 Hz, 1H, CH–CH2–O), 4.03 (s, 1H, CH–CH–S2), 3.78 (s, 3H, 4′-OCH3), 3.73 (s, 6H, 3′,5′-OCH3), 2.86 (dd, J = 21.3, 14.4 Hz, 5H, O
O), 4.57 (d, J = 3.1 Hz, 1H, CH-Ar), 4.34 (t, J = 7.7 Hz, 1H, CH–CH2–O), 4.17 (t, J = 9.6 Hz, 1H, CH–CH2–O), 4.03 (s, 1H, CH–CH–S2), 3.78 (s, 3H, 4′-OCH3), 3.73 (s, 6H, 3′,5′-OCH3), 2.86 (dd, J = 21.3, 14.4 Hz, 5H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH, O–CH2–CH, CH2–S, CH–S), 2.40 (s, 2H, CH2–C
C–CH, O–CH2–CH, CH2–S, CH–S), 2.40 (s, 2H, CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2.08 (d, J = 12.9 Hz, 1H, S–CH2–CH2), 1.95–1.42 (m, 18H, S–CH2–CH2, S–CH–CH2–CH2–CH2, CH–H). 13C NMR (126 MHz, CDCl3) δ 173.89 (12-C), 173.60 (14-C), 152.61 (3′,5′-C), 148.10 (7-C), 147.57 (6-C), 137.15 (4′-C), 134.81 (1′-C), 132.34 (9-C), 128.36 (10-C), 109.70 (8-C), 108.11 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.48 (4-C), 71.34 (11-C), 60.71 (4′-OCH3), 56.32 (3′,5′-OCH3), 56.14 (22-C), 45.52 (2-C), 45.00 (1-C), 43.72 (19-C), 43.16 (1′′-C), 38.72 (3-C), 34.13 (15-C), 33.94 (18-C), 31.32 (20-C), 30.55 (2′′,6′′-C), 30.42 (16-C), 26.24 (4′′-C), 26.15 (3′′,5′′-C), 25.71 (17-C), 24.85 (21-C). ESI-TOF, calcd for C37H46O9S2 ([M + Na]+) 721.2583, found 721.1322. Anal. calcd for C37H46O9S2: C, 63.59; H, 6.63; O, 20.60; S, 9.18%. Found: C, 62.55; H, 7.11; O, 21.17; S, 9.11%.
O), 2.08 (d, J = 12.9 Hz, 1H, S–CH2–CH2), 1.95–1.42 (m, 18H, S–CH2–CH2, S–CH–CH2–CH2–CH2, CH–H). 13C NMR (126 MHz, CDCl3) δ 173.89 (12-C), 173.60 (14-C), 152.61 (3′,5′-C), 148.10 (7-C), 147.57 (6-C), 137.15 (4′-C), 134.81 (1′-C), 132.34 (9-C), 128.36 (10-C), 109.70 (8-C), 108.11 (5-C), 106.95 (2′,6′-C), 101.59 (13-C), 73.48 (4-C), 71.34 (11-C), 60.71 (4′-OCH3), 56.32 (3′,5′-OCH3), 56.14 (22-C), 45.52 (2-C), 45.00 (1-C), 43.72 (19-C), 43.16 (1′′-C), 38.72 (3-C), 34.13 (15-C), 33.94 (18-C), 31.32 (20-C), 30.55 (2′′,6′′-C), 30.42 (16-C), 26.24 (4′′-C), 26.15 (3′′,5′′-C), 25.71 (17-C), 24.85 (21-C). ESI-TOF, calcd for C37H46O9S2 ([M + Na]+) 721.2583, found 721.1322. Anal. calcd for C37H46O9S2: C, 63.59; H, 6.63; O, 20.60; S, 9.18%. Found: C, 62.55; H, 7.11; O, 21.17; S, 9.11%.Cell lines and culture conditions
HeLa, A549, Calu-1, L02 and Vero cells were purchased from Nanjing Keygen Technology (Nanjing, China). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Hyclone) (high glucose) with L-glutamine supplemented with 10% fetal bovine serum (FBS, Transgene), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Hyclone), and incubated at 37 °C in a humidified atmosphere containing 5% CO2.Antiproliferation assay
Cancer cell lines were grown to log phase in DMEM medium supplemented with 10% fetal bovine serum. After diluting to 2 × 104 cells per mL with DMEM medium, 100 μL of the obtained cell suspension was added to each well of 96-well culture plates and then allowed to adhere for 12 h at 37 °C, 5% CO2 atmosphere. Tested samples at various concentrations (0.1, 1, 10, and 100 μM) were added to the 96-well culture plates with podophyllotoxin and CA-4 as positive references.After 24 h, 20 μL of PBS containing 2.5 mg mL−1 of MTT was added to each well. Plates were then incubated for further 4 h, and then were centrifuged (1500 rpm at 4 °C for 10 min) to remove supernatant. Next, 150 μL of DMSO was added to each well for coloration. The plates were shaken gently to ensure complete solubilisation of formazan for 10 min at room temperature. The absorbance was measured and recorded on an ELISA reader (EPOCH, BioTek, USA) at a test wavelength of 570 nm. In all experiments three replicate wells were used for each drug concentration. Each assay was carried out at least three times and the results were shown in Table 2.
Cell apoptosis assay
Briefly, 5 × 103 cells were seeded in 6-well plates for 24 h and then were treated with L4 (0, 1, 3, and 9 μM) for 24 h. Then cells were collected and washed twice with PBS and stained with 5 μL of Annexin V-FITC and 2.5 μL of PI (5 μg mL−1) in 1× binding buffer (10 mM HEPES, pH 7.4, 140 mM NaOH, 2.5 mM CaCl2) for 15 min at room temperature in the dark. Apoptotic cells were quantified using a BD Accuri C6 Flow Cytometer (BD, USA). Statistical analysis was done using Flowjo 7.6.1 software. Both early apoptotic (Annexin V-positive, PI-negative) and late apoptotic (double positive of Annexin V and PI) cells were detected.Cell cycle analysis
A549 cells were plated in 6-well plates at 5.0 × 103 cells per well and incubated at 37 °C for 12 h. Exponentially growing cells were then incubated with the L4 at 0, 0.4, 0.8, and 1.6 μM for 8 h. And in the time-dependent assays, exponentially growing cells were incubated with 0.8 μM L4 at 37 °C for 4, 8, and 16 h. After then, cells were centrifuged and fixed in 70% ethanol at −20 °C for at least 12 h and subsequently resuspended in PBS containing 0.1 mg mL−1 RNase A and 5 μg mL−1 propidium iodide (PI). Cellular DNA content, for cell cycle distribution analysis, was measured by flow cytometry using BD Accuri C6 Flow Cytometer (BD, USA) plotting 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events per sample. The percentage of cells in the G1, S and G2/M phases of the cell cycle were determined using the Flowjo 7.6.1 software after cell debris exclusion.
000 events per sample. The percentage of cells in the G1, S and G2/M phases of the cell cycle were determined using the Flowjo 7.6.1 software after cell debris exclusion.
Confocal microscopy assay
A549 cells were grown on glass bottom cell culture dish (NEST, Wuxi, China) to 70% confluence and incubated with 1.5 μM of L4, 2 μM of colchicine and 2 μM of taxol for 12 h, respectively. After incubating, cells were washed three times with PBS and fixed with 4% paraformaldehyde for 20 min, permeabilized with 1% TritonX-100 for another 10 min. Then, the cells were blocked with 3% BSA for 1 h at room temperature. Subsequently, the cells were washed once with PBS, and incubated with anti-tubulin antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Cytoskeleton, Inc.) in 3% BSA overnight at 4 °C. After being washed with PBS for three times, each coverslip was added 200 μL of Cy3-labeled goat anti-mouse IgG (H + L) (1
500, Cytoskeleton, Inc.) in 3% BSA overnight at 4 °C. After being washed with PBS for three times, each coverslip was added 200 μL of Cy3-labeled goat anti-mouse IgG (H + L) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, Cytoskeleton, Inc.) in 3% BSA and incubated for 1 h at room temperature followed by DAPI (5 ng mL−1). Cells were then observed under an Olympus confocal microscope.
1500, Cytoskeleton, Inc.) in 3% BSA and incubated for 1 h at room temperature followed by DAPI (5 ng mL−1). Cells were then observed under an Olympus confocal microscope.
In vitro microtubule assembly assay
We used an established method to measure soluble (depolymerized) and assembled (polymerized) tubulin.26 A549 cells (5 × 107 per flask) were seeded in 75 T flask and exposed to taxol (2 μM), colchicine (2 μM), and L4 (1.5 μM) for 16 h. After treatment, cells were collected and washed twice with PBS then lysed at 37 °C for 5 min with 50 μL of hypotonic buffer (1 mM MgCl2, 2 mM EGTA, 0.5% NP-40, 2 mM PMSF, 200 units mL−1 aprotinin, 5 mM amino caproic acid, 1 mM benzamidine, and 20 mM Tris–HCl, pH 6.8). The cell lysates were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 25 °C. The supernatants containing soluble (cytosolic) tubulin were separated from the pellets containing polymerized (cytoskeletal) tubulin. The pellets were resuspended in 100 μL of hypotonic buffer, sonicated on ice, mixed with 5× sample buffer, and heated for 5 min at 100 °C. Equal amounts of the two fractions were partitioned by SDS-polyacrylamide gel electrophoresis. Immunoblots were probed with β-tubulin monoclonal antibody and secondary HRP-conjugated antibody. The blots were developed by using an ECL kit and Kodak Bio-MAX MR film (Eastman Kodak, Rochester, NY). All results are from three independent experiments.
000 rpm for 10 min at 25 °C. The supernatants containing soluble (cytosolic) tubulin were separated from the pellets containing polymerized (cytoskeletal) tubulin. The pellets were resuspended in 100 μL of hypotonic buffer, sonicated on ice, mixed with 5× sample buffer, and heated for 5 min at 100 °C. Equal amounts of the two fractions were partitioned by SDS-polyacrylamide gel electrophoresis. Immunoblots were probed with β-tubulin monoclonal antibody and secondary HRP-conjugated antibody. The blots were developed by using an ECL kit and Kodak Bio-MAX MR film (Eastman Kodak, Rochester, NY). All results are from three independent experiments.
Conclusion
In summary, we synthesized a series of aryl dithian valeryl podophyllotoxin ester derivatives and evaluated their cytotoxic activity against three cancer cell lines and two non-cancer cell lines. The introduction of lipoyl moiety to podophyllotoxin scaffold reduced the cytotoxicity of podophyllotoxin toward the non-cancer cells and improved its tumor targeting. Among them, L4 showed the best antiproliferation activity against A549 and the effect was similar to that of CA-4, which was the positive control in this study. The following flow cytometry analysis results demonstrated that L4 can potently lead to cell cycle arrest at G2/M phase but the effect on apoptosis inducing was not very significant. Furthermore, confocal microscopy and western blot analysis results suggested that L4 can potently inhibit tubulin polymerization, thus causing cell cycle arrest and mitosis disruption.Abbreviations
| MTT | (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) |
| CA-4 | Combretastatin A-4 |
| NMR | Nuclear magnetic resonance spectrum |
| TLC | Thin layer chromatography |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| BSA | Bovine serum albumin |
| EGTA | Ethylenebis (oxyethylenenitrilo) tetraacetic acid |
| PMSF | Phenylmethane sulfonyl fluoride |
Acknowledgements
The authors are grateful to the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R27).Notes and references
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253–265 CrossRef CAS PubMed.
- K. Ahmed, V. S. Reddy, V. Santhosh and S. A. Basha, Org. Biomol. Chem., 2015, 13, 3416–3431 Search PubMed.
- A. Kamal, M. K. Reddy, T. B. Shaik, Rajender, Y. V. V. Srikanth, V. S. Reddy, G. B. Kumar and S. V. Kalivendi, Eur. J. Med. Chem., 2012, 50, 9–17 CrossRef CAS PubMed.
- A. Kamal, A. B. Shaik, N. Jain, C. Kishor, A. Nagabhushana, B. Supriya, G. B. Kumar, S. S. Chourasiya, Y. Suresh, R. K. Mishra and A. Addlagatta, Eur. J. Med. Chem., 2015, 92, 501–513 CrossRef CAS PubMed.
- A. Kamal, A. B. Shaik, S. Polepalli, G. B. Kumar, V. S. Reddy, R. Mahesh, S. Garimella and N. Jain, Bioorg. Med. Chem., 2015, 23, 1082–1095 CrossRef CAS PubMed.
- S. Santoshi, N. K. Manchukonda, C. Suri, M. Sharma, B. Sridhar, S. Joseph, M. Lopus, S. Kantevari, I. Baitharu and P. K. Naik, J. Comput.-Aided Mol. Des., 2015, 29, 249–270 CrossRef CAS PubMed.
- M. A. Gregory, C. L. Gabriel, H. K. Elizabeth, R. Zhang, B. David and N. Eva, Cell, 2014, 157, 1117–1129 CrossRef PubMed.
- M. A. Jordan, K. Wendell, S. Gardiner, W. B. Derry, H. Copp and L. Wilson, Cancer Res., 1996, 56, 816–825 CAS.
- D. Y. Zuo, D. D. Guo, X. W. Jiang, Q. Guan, H. Qi, J. W. Xu, Z. Q. Li, F. S. Yang, W. G. Zhang and Y. L. Wu, Chem.-Biol. Interact., 2015, 227, 7–17 CrossRef CAS PubMed.
- E. S. Garcia and P. Azambuja, Toxicon, 2004, 44, 431–459 CrossRef CAS PubMed.
- I. Jardine, Natural products as leads to anticancer drugs, Academic Press, New York, 1980, pp. 319–351 Search PubMed.
- B. Umesha, Y. B. Basavaraju and C. Mahendra, Med. Chem. Res., 2015, 24, 142–151 CrossRef CAS.
- L. Yang, X. Nan, W. Q. Li, M. J. Wang, X. B. Zhao, Y. Q. Liu, Z. J. Zhang and K. H. Lee, Med. Chem. Res., 2014, 23, 4926–4931 CrossRef CAS PubMed.
- M. A. Shareef, D. Duscharla, G. Ramasatyaveni, N. R. Dhoke, A. Das, R. Ummanni and A. K. Srivastava, Eur. J. Med. Chem., 2015, 89, 128–137 CrossRef CAS PubMed.
- X. Y. Zhi, C. Yang, X. Yu and H. Xu, Bioorg. Med. Chem. Lett., 2014, 24, 5679–5682 CrossRef CAS PubMed.
- M. K. Zilla, D. Nayak, H. Amin, Y. Nalli, B. Rah, S. Chakraborty, S. Kitchlu, A. Goswami and A. Ali, Chem.-Biol. Interact., 2014, 224, 100–107 CrossRef CAS PubMed.
- A. A. Yadav, X. Wu, D. Patel, J. C. Yalowich and B. B. Hasinoff, Bioorg. Med. Chem., 2014, 22, 5935–5949 CrossRef CAS PubMed.
- H. Wang, L. J. Tang, Y. J. Tang and Z. P. Yuan, Bioorg. Med. Chem., 2014, 22, 6183–6192 CrossRef CAS PubMed.
- W. H. Cheng, B. Cao, H. Shang, C. Niu, L. M. Zhang, Z. H. Zhang, D. L. Tian, S. Zhang, H. Chen and Z. M. Zou, Eur. J. Med. Chem., 2014, 85, 498–507 CrossRef CAS PubMed.
- Y. Q. Liu, J. Tian, K. Qian, X. B. Zhao, S. L. M. Natschke, L. Yang, X. Nan, X. Tian and K. H. Lee, Med. Res. Rev., 2015, 35, 1–62 CrossRef PubMed.
- H. Wang, L. J. Tang, Y. J. Tang and Z. P. Yuana, Bioorg. Med. Chem., 2014, 22, 6183–6192 CrossRef CAS PubMed.
- J. Moungjaroen, U. Nimmannit, P. S. Callery, L. Y. Wang, N. Azad, V. Lipipun, P. Chanvorachote and Y. Rojanasakul, J. Pharmacol. Exp. Ther., 2006, 319, 1062–1069 CrossRef CAS PubMed.
- E. Selvakumar, C. Prahalathan, P. T. Sudharsan and P. Varalakshmi, Toxicology, 2006, 217, 71–78 CrossRef CAS PubMed.
- E. Dozio, M. Ruscica, L. Passafaro, G. Dogliotti, L. Steffani, A. Pagani, G. Demartini, D. Esposti, F. Fraschini and P. Magni, Eur. J. Pharmacol., 2010, 641, 29–34 CrossRef CAS PubMed.
- R. R. Wei, L. Cheng, M. Zheng, R. Cheng, F. H. Meng, C. Deng and Z. Y. Zhong, Biomacromolecules, 2012, 13, 2429–2438 CrossRef CAS PubMed.
- S. W. Wang, S. L. Pan, Y. C. Huang, J. H. Guh, P. C. Chiang, D. Y. Huang, S. C. Kuo, K. H. Lee and C. M. Teng, Mol. Cancer Ther., 2008, 7, 350–360 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04902d |
| ‡ These two authors equally contribute to this paper. |
| This journal is © The Royal Society of Chemistry 2015 |