High apparent strengthening efficiency for reduced graphene oxide in copper matrix composites produced by molecule-lever mixing and high-shear mixing
Abstract
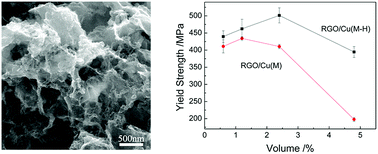
Reduced graphene oxide (RGO) reinforced copper matrix composites (RGO/Cu composites) with volume fractions of RGO from 0.6 to 4.8 vol.% were produced based on a molecular-level mixing method (MLM). High-shear mixing was introduced in the process of MLM by using a rotor-stator mixer, which could make the RGO sheets distributed in the composite more homogeneous and improve the properties of the composites. The MLM method integrated with high-shear mixing is abbreviated as M-H. The effect of high-shear mixing on the mechanical properties of the composites with different volume fractions of graphene was studied. The yield strength of the 2.4 vol.% RGO/Cu composite produced by M-H method is 501.3 MPa, which is more than three times higher than that of the Cu matrix. RGO shows extremely high strengthening effect; the apparent strengthening efficiency of RGO in the 0.6 vol.% RGO/Cu composite is as high as 321.7, even higher than CNTs. The results show that the M-H method is hopeful to be applied to produce many kinds of graphene based composites.

 Please wait while we load your content...
Please wait while we load your content...