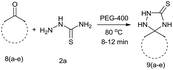
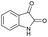
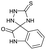
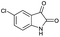
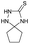PEG-assisted two-component approach for the facile synthesis of 5-aryl-1,2,4-triazolidine-3-thiones under catalyst-free conditions†
Rathinam Ramesh and
Appaswami Lalitha*
Department of Chemistry, Periyar University, Periyar Palkalai Nagar, Salem-636011, Tamil Nadu, India. E-mail: lalitha2531@yahoo.co.in; Fax: +91-427-2345124; Tel: +91-427-2345271
First published on 2nd June 2015
Abstract
A straightforward and greener PEG-assisted protocol has been disclosed for the facile synthesis of 5-aryl-1,2,4-triazolidine-3-thiones via the two-component reaction of multifarious aromatic aldehydes and thiosemicarbazides using very convenient neutral reaction conditions. This methodology represents a sustainable approach for rapid access to a library of diversity-oriented highly pure triazolidine scaffolds with broad substrate scope.
The exploration of environmentally amenable synthetic routes for privileged ‘drug-like’ heterocyclic candidates from readily accessible precursors under the classification of green chemistry is gaining substantial interest in areas such as pharmaceuticals, academia and industry.1 In recent years, synthetic chemists have aimed to replace toxic and volatile organic solvents as reaction media with sustainable alternatives such as water, PEG and ionic liquids, or carrying out reactions under solvent-free conditions.2 In addition, catalyst-free synthetic approaches are a remarkable tool in scientific society because they are associated with minimization of pollution, cost and purity-related problems.3 The use of polyethylene glycol (PEG) as a greener alternative in organic synthesis has become more favourable over toxic organic solvents due to its non-toxicity, bio-compatibility, bio-degradability and miscibility with both aqueous and several non-aqueous solvents.4 Numerous PEG-mediated organic transformations have been reported, including oxidation and reduction reactions,5 the Heck reaction,6 substitution reactions,7 the Suzuki cross-coupling reaction,8 partial reductions of alkynes,9 asymmetric dihydroxylation10 and the Wacker reaction.11 The hetero-cyclizations of readily available linear compounds is one of the most common and admired lines of attack for synthesizing valuable heterocycles. Very fascinatingly, several natural and synthetic molecules bearing heterocyclic moieties have been documented as potential drug materials with extensive biological activities.12 Among these, nitrogen-containing heterocyclic compounds and their analogues, present in natural as well as synthetic biologically active molecules, are pharmaceutically imperative.
Triazole is an important five-membered heterocyclic motif with three nitrogen and two carbon atoms, which is extensively employed in several industries of pharmaceutical interest.13 In particular, the chemistry of 1,2,4-triazoles plays an essential role and their fused heterocyclic motifs are often found in several anticonvulsant14 and oncological drug materials.15 The 1,2,4-triazole functionality has been incorporated into a range of therapeutically interesting drugs, including central nervous system (CNS) stimulants, sedatives, H1/H2 histamine receptor blockers, anticonvulsants, analgesics, anti-anxiety and cholinesterase active agents.16–19 The 1,2,4-triazoles bearing thione moieties have been well studied and have an array of biological activities such as anti-cancer,20 anti-HIV,21 anti-viral,22 antidepressant,23 cytotoxic,24 antiepileptic,25 anti-allergic,26 analgesic27 and antitubercular.28 Moreover, these derivatives have been used very much in agrochemicals due to their plant growth regulatory action.29 1,2,4-Triazolidine-3-thione and its derivatives can be conveniently converted into biologically potent 1,2,4-triazoles.30 To the best of our knowledge, a very limited number of synthetic methods have been reported in the literature for the preparation of 5-aryl-1,2,4-triazolidine-3-thiones.31 In this paper, we wish to report an expeditious and atom economic PEG-400-assisted two-component reaction for the synthesis of diverse 1,2,4-triazolidine-3-thione derivatives as potential building blocks under catalyst-free conditions from different substituted aldehydes and thiosemicarbazides.
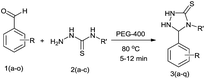
In our preliminary attempt, we have examined the reaction of 4-chlorobenzaldehyde (1b) with thiosemicarbazide (2a) in ethanol as a simple model strategy. The reaction mixture was stirred at reflux temperature for 40 min, which afforded 79% of product as a white crystalline solid. The same reaction has also been studied with different solvents, like methanol, 2-propanol and water at reflux temperature, from which the results were moderate (68–75% yield). These experiments showed that the condensation between the aromatic aldehyde and thiosemicarbazide took place smoothly even in the absence of any third-component as a catalyst. It was interesting that the same reaction gave an excellent result (94% yield) within 7 min when using PEG-400 as an eco-friendly medium at 75 °C. It was found that the reaction proceeded very well and with increased yields, which clearly indicate that the PEG was the most effective promoter for this transformation. From the results depicted in Table 1, we have selected polyethylene glycol as a suitable reaction medium due to the reaction time, yield and environmental compatibility.
| Entry | Solvent | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (2.0 mmol), thiosemicarbazide (2.0 mmol) and solvent (2.0 ml) stirred at reflux temperature.b Isolated yield of 3b.c Isolated yield of 3b using PEG-400 (0.5 ml) at 75 °C. | |||
| 1 | Ethanol | 40 | 79 |
| 2 | Methanol | 40 | 74 |
| 3 | 2-Propanol | 45 | 68 |
| 4 | Water | 30 | 75 |
| 5 | PEG-400 | 7 | 94c |
A study of the effect of temperature on the reaction time as well as on the yield of the product revealed that the reaction was strongly influenced by the temperature. When the reaction was carried out at room temperature, the yield was found to be only 66% after 45 min with PEG-400 as the solvent. Screening the results of Table 2 revealed that 80 °C would be optimal temperature, at which the reaction proceeded rapidly and produced the best yield (97%) of 3b in 6 min. A further increase in the temperature did not cause any significant change in the product yield and time of the reaction.
The versatility of this method was evaluated by investigating the substrate scope using the conditions outlined in Scheme 1.32 A variety of substituted aldehydes were reacted under the above optimized reaction conditions to afford good to high yields of the corresponding 5-aryl-1,2,4-triazolidine-3-thiones within a reasonable time frame, and the results are summarized in Table 3. Aromatic aldehydes bearing electron donating (entries 5, 7, 8, 10, 12 and 14) and withdrawing (2, 3, 4, 6, 11, 13 and 15) groups were all converted quickly into their corresponding triazolidine-3-thiones, with excellent yields of 94–97%. From the results, we conclude that there is no substituent effect and the studied reactions were completed with very short reaction times, with negligible deviations. Similarly, methyl and phenyl substituted thiosemicarbazides can be utilized as substrates for this protocol, from which ∼95% yields of the N-substituted 1,2,4-triazolidine-3-thione derivatives are obtained with excellent purity. It is worth mentioning that the work-up of this reaction is very easy and requires only simple filtration and washing the residue with water to provide highly pure target molecules, the structures of which were confirmed by IR, 1H and 13C NMR and elemental analysis.
| Entry | R′ | Aldehyde | Product | Time (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aromatic aldehyde (2.0 mmol), thiosemicarbazide (2.0 mmol) and PEG (0.5 ml) stirred at 80 °C.b Reaction conditions: terephthalaldehyde (2.0 mmol), thiosemicarbazide (4.0 mmol) and PEG (0.5 ml) stirred at 80 °C.c Isolated yield.d Novel compounds. | |||||
| 1 | H | 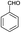 |
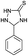 |
7 | 96 (ref. 31a) |
| 2 | H |  |
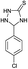 |
6 | 97 (ref. 31a) |
| 3 | H |  |
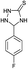 |
6 | 95d |
| 4 | H |  |
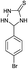 |
7 | 96d |
| 5 | H |  |
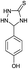 |
8 | 95d |
| 6 | H |  |
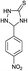 |
8 | 96d |
| 7 | H | 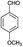 |
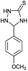 |
10 | 95 (ref. 31a) |
| 8 | H |  |
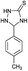 |
12 | 96 (ref. 31b) |
| 9 | H |  |
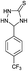 |
10 | 94d |
| 10 | H | 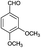 |
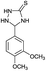 |
12 | 95 (ref. 31b) |
| 11 | H |  |
 |
5 | 96d |
| 12 | H |  |
 |
12 | 95 (ref. 31b) |
| 13 | H | 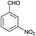 |
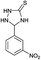 |
10 | 95d |
| 14 | H | 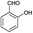 |
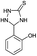 |
8 | 94d |
| 15 | H | 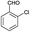 |
 |
6 | 95d |
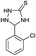
| 16 | CH3 |  |
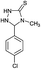 |
6 | 95 (ref. 31a) |
| 17 | C6H5 |  |
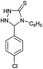 |
6 | 93d |
| 18b | H |  |
 |
8 | 96 (ref. 31a) |
| 19b | CH3 |  |
 |
10 | 95d |
| 20b | C6H5 |  |
 |
10 | 95d |
The IR spectrum of compound 3b (Table 3, entry 2) shows dissimilar absorption bands at 3435, 3282 and 3165 cm−1 which indicate the presence of three –NH stretching vibrations. In addition, a band at 1282 cm−1 shows the existence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching. The 1H NMR spectrum exhibited three notable singlets at δ 11.49, 8.24, and 8.02 ppm confirming the presence of three –NH protons and a singlet at δ 8.08 ppm indicating the presence of a benzylic methine proton. In the 13C NMR, the signal at δ 178 ppm indicates the presence of a thiocarbonyl moiety, which also helped to confirm the formation of 3b.
S stretching. The 1H NMR spectrum exhibited three notable singlets at δ 11.49, 8.24, and 8.02 ppm confirming the presence of three –NH protons and a singlet at δ 8.08 ppm indicating the presence of a benzylic methine proton. In the 13C NMR, the signal at δ 178 ppm indicates the presence of a thiocarbonyl moiety, which also helped to confirm the formation of 3b.
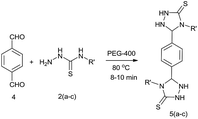
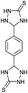
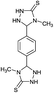
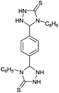
The effect of the bulkiness of the various substituents on the aromatic ring did not reduce the reactivity towards the formation of triazolidine derivatives. The reactions worked well with aldehydes containing substituents at the ortho position (Table 3, entries 11, 14 and 15). Next, under the optimized conditions, the scope of the present protocol was investigated by using a bis-aldehyde, namely terephthalaldehyde (Scheme 2). We were delighted to observe that the reaction of terephthaldehyde (4) with different substituted thiosemicarbazides (2a–c) under the optimal conditions furnished 1,4-phenylene-bis-1,2,4-triazolidine-3-thiones (Table 3, entries 18–20, 95%) in good yields.
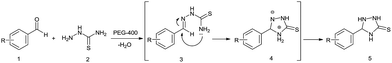
From the above results, the mechanistic route of the present two-component reaction can be envisioned (Scheme 3) as the initial formation of the intermediate 3 by the nucleophilic addition of thiosemicarbazide (2) to the carbonyl carbon of aldehyde (1), followed by an intra-molecular nucleophilic attack of the –NH2 of 3 at the azomethine carbon at 80 °C, to yield the desired 5-aryl-1,2,4-triazolidine-3-thiones.
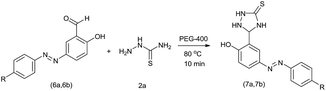
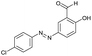
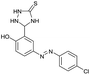
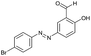
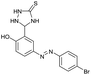
The extensive tolerance of the functional groups present on the aromatic aldehydes stimulated us to extend our study to some azo-based diazenyl-2-hydroxybenzaldehyde derivatives (Table 4). The 5-(4-chloro/bromophenyl)diazenyl-2-hydroxybenzaldehydes (6a, 6b) have been treated with thiosemicarbazide (2a) under the same optimized thermal conditions, where ∼93% yields of the novel 5-(2-hydroxy-5-phenyldiazenylphenyl)-1,2,4-triazolidine-3-thiones (7a, 7b) were obtained in 10 min.
The generality of the present protocol was further studied by the utilization of several cyclic ketones instead of aromatic aldehydes. Notably, these reactions proceeded very smoothly, leading to excellent yields, and the results are depicted in Table 5. It is of value to note that the substituted 1,2,4-triaza-3-thione derivatives have been achieved in good yields with an interesting spiro-centre via simple green transformations providing access to bicyclic units in a highly efficient manner.
The formation of the novel 1,2,4-triazaspiro[4.4]nonane-3-thione (Table 5, entry 2) was confirmed using various spectral techniques. The 1H NMR shows different singlets at δ 7.47, 7.96 and 9.82 ppm, indicating the presence of three –NH protons, along with multiplets at δ 1.61 and 2.28 ppm, which correspond to the aliphatic methylene moiety. In the 13C NMR, peaks at δ 178 and 163 ppm clearly denote the existence of thiocarbonyl and spiro-centered carbon atoms.
The results from all of the above studies clearly indicate that the present non-catalytic protocol should be extendable to a wide range of substrates to construct a diversity-oriented library of 1,2,4-triazolidine-3-thiones.
In conclusion, we have developed a convergent and robust greener protocol for the synthesis of structurally diverse 1,2,4-triazolidine-3-thione derivatives by the two-component reaction of various aldehydes or cyclic ketones with thiosemicarbazides using PEG-400 as an effective promoter under catalyst-free conditions. The simple, straightforward, atom-economic, rapid, high yielding as well as inexpensive and eco-friendly nature are the key benefits of this method, which constitutes an attractive tool addressing the access to 1,2,4-triazolidine-3-thiones for industrial applications.
Acknowledgements
R.R. gratefully thanks the DST-Inspire Fellowship, New Delhi, India (no. DST/INSPIRE Fellowship/2012/690) for financial assistance.Notes and references
-
(a) P. T. Anastas, J. C. Warner, Green Chemistry, Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed
; (b) J. Clark, D. M. A. Macquarrie, Handbook of Green Chemistry & Technology, Blackwell, Oxford, 2002 Search PubMed
.
- F. Toda and K. Tanaka, Chem. Rev., 2000, 100, 1025 CrossRef PubMed
.
- M. Chennapuram, N. R. Emmadi, C. Bingi, J. B. Nanubolub and K. Atmakur, Green Chem., 2014, 16, 3237 RSC
.
- Z. H. Zhang, L. Yin, Y. M. Wang, J. Y. Liu and Y. Li, Green Chem., 2004, 6, 563 RSC
.
- J. R. Blanton, Synth. Commun., 1997, 27, 2093 CrossRef CAS PubMed
.
- S. Chandrasekhar, C. Narsihmulu, S. S. Sultana and N. R. K. Reddy, Org. Lett., 2002, 4, 4399 CrossRef CAS PubMed
.
- P. Ferravoschi, A. Fiecchi, P. Grisenti, E. Santaniello and S. Trave, Synth. Commun., 1987, 17, 1569 CrossRef PubMed
.
- V. V. Namboodiri and R. S. Varma, Green Chem., 2001, 3, 146 RSC
.
- S. Chandrasekhar, C. Narsihmulu, G. Chandrasekhar and T. Shyamsundar, Tetrahedron Lett., 2004, 45, 2421 CrossRef CAS PubMed
.
- S. Chandrasekhar, C. Narsihmulu, S. S. Sultana and N. R. K. Reddy, Chem. Commun., 2003, 1716 RSC
.
- A. Haimov and R. Neumann, Chem. Commun., 2002, 876 RSC
.
-
(a) C. Bailly, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 363 CrossRef CAS
; (b) H. Fan, J. Peng, M. T. Hamann and J. F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed
.
- A. Masoudi, Y. A. Soud, N. J. Salihi and N. A. Masoudi, Chem. Heterocycl. Compd., 2006, 42, 1377 CrossRef PubMed
.
- G. A. R. Johnston, Curr. Top. Med. Chem., 2002, 2, 903 CrossRef CAS
.
- M. Clemons, R. E. Coleman and S. Verma, Cancer Treat. Rev., 2004, 30, 325 CrossRef CAS PubMed
.
- N. D. Heindel and J. R. Reid, J. Heterocycl. Chem., 1980, 17, 1087 CrossRef CAS PubMed
.
- R. A. Glennon, M. R. Seggel, W. H. Sonie, K. H. Davis, R. A. Lyon and M. Titeler, J. Med. Chem., 1988, 31, 5 CrossRef CAS
.
- S. Stankovsky, F. Jedlovska and K. Spirkova, Collect. Czech. Chem. Commun., 1993, 58, 2211 CrossRef CAS
.
- M. Z. Krimer, V. P. Tashch, Y. B. Kalyan, F. Z. Bakaev, Y. G. Putskin and Y. Molchanov, Khim.-Farm. Zh., 1995, 29, 764 Search PubMed
.
-
(a) F. Pagliai, T. Pirali, E. D. Grosso, R. D. Brisco, G. C. Tron, G. Sorba and A. A. Genazzani, J. Med. Chem., 2006, 49, 467 CrossRef CAS PubMed
; (b) S. A. Bakunov, S. M. Bakunova, T. Wenzler, M. Ghebru, K. A. Werbovetz, R. Brun and R. R. Tidwell, J. Med. Chem., 2010, 53, 254 CrossRef CAS PubMed
; (c) A. H. Banday, S. A. Shameem, B. D. Gupta and H. M. Sampath Kumar, Steroids, 2010, 75, 801 CrossRef CAS PubMed
.
- R. Alvarez, S. Velazquez, A. S. Felix, S. Aquaro, E. D. Clercq, C. F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4194 CrossRef
.
- J. T. Witkowski, R. K. Robins, G. P. Khare and R. W. Sidwell, J. Med. Chem., 1973, 16, 935 CrossRef CAS
.
- S. G. Umut, G. K. Nesrin, G. Ozgur, K. Yavuz, K. Ekrem and I. Samil, Bioorg. Med. Chem., 2007, 15, 5738 CrossRef PubMed
.
- N. Demirbas, S. A. Karaoglu, A. Demirbas and K. Sancak, Eur. J. Med. Chem., 2004, 39, 793 CrossRef CAS PubMed
.
- S. Hakimian, A. C. Hakimian, G. D. Anderson and J. W. Miller, Expert Opin. Pharmacother., 2007, 8, 1931 CrossRef CAS PubMed
.
- D. R. Buckle, C. J. M. Rockell, H. Smith and B. A. Spicer, J. Med. Chem., 1986, 29, 226 CrossRef
.
- J. Y. Jin, L. X. Zhang, A. J. Zhang, X. X. Lei and J. H. Zhu, Molecules, 2007, 12, 1596 CrossRef CAS
.
- A. A. Yaseen, N. A. Mohammad and A. A. Najim, Rev. Bras. Farmacogn., 2004, 59, 775 Search PubMed
.
- U. M. Wagner, H. K. Reitze and K. A. Seitz, Acarolog., 1990, 8, 27 CrossRef
.
-
(a) I. M. Lagoja, C. Pannecouque, L. Musumeci, M. Froeyen, A. V. Aerschot, J. Balzarini, P. Herdewijn and E. D. Clercq, Helv. Chim. Acta, 2002, 85, 1883 CrossRef CAS
; (b) J. G. Schantl, S. Lang and K. Wurst, Heterocycles, 1999, 50, 251 CrossRef CAS PubMed
; (c) S. D. Ziman, J. Heterocycl. Chem., 1980, 17, 1319 CrossRef CAS PubMed
; (d) L. Labanauskas, E. Udrenaite, P. Gaidelis and A. Brukstus, Rev. Bras. Farmacogn., 2004, 59, 255 CAS
.
-
(a) J. D. Patil and D. M. Pore, RSC Adv., 2014, 4, 14314 RSC
; (b) M. M. Mane and D. M. Pore, Tetrahedron Lett., 2014, 55, 6601 CrossRef CAS PubMed
; (c) D. M. Pore, P. G. Hegade, M. M. Mane and J. D. Patil, RSC Adv., 2013, 3, 25723 RSC
.
- Typical procedure for the synthesis of 5-aryl-1,2,4-triazolidine-3-thione derivatives: a mixture of aromatic aldehyde or cyclic ketone (2.0 mmol) and thiosemicarbazide (2.0 mmol) was added to 0.5 ml of PEG-400. The resulting mixture was allowed to stir at 80 °C for the time specified in Tables 3–5. After completion of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature and treated with de-ionized water (10 ml), well-stirred for a few minutes, and the generated solid was filtered off. The crude product was recrystallized from 95% ethanol to afford the corresponding highly pure 1,2,4-triazolidine-3-thione derivatives. The recovered PEG can be reused for a further two cycles with negligible loss of its efficiency.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07726e |
| This journal is © The Royal Society of Chemistry 2015 |