Chiral liquid membrane for enantioselective separation of racemic ibuprofen by L-tartaric acid derivatives†
Fan Zhang,
Lichao He,
Wei Sun,
Yongqi Cheng,
Junteng Liu and
Zhongqi Ren*
Beijing Key Laboratory of Membrane Science and Technology, Beijing University of Chemical Technology, NO. 15, N. 3rd Ring Rd East, Beijing 100029, People's Republic of China. E-mail: renzq@mail.buct.edu.cn; Fax: +86-10-6443-6781; Tel: +86-10-6442-3628
First published on 27th April 2015
Abstract
The chirality of drugs plays a significant role in most chemical and biochemical process. In this paper, a chiral liquid membrane using L-tartaric ester dissolved in n-octane as liquid membrane phase and polyvinylidene fluoride hollow fibers as membrane support was investigated to separate racemic ibuprofen. For L-dipentyl tartaric ester, the separation factor was 1.18. The favorable L-dipentyl tartaric ester concentration was 0.20 mol L−1. With an increase of flow rates on two sides, a flux change of mass transfer in stripping phase was not observed. The same trend is obtained in feed phase. The concentration of both R-ibuprofen and S-ibuprofen in stripping phase increased with an increase of pH value. The best pH in stripping phase was 2.5 and the separation factor was about 1.2. The best separation factor was up to 1.38 after a six-level experiment.
1. Introduction
Ibuprofen ((R,S)-α-methyl-4-(2-methylpropyl)benzeneacetic acid) is a nonsteroidal anti-inflammatory drug (NSAID) and has been used as an anti-inflammatory and antipyretic agent for the treatment of rheumatoid arthritis, degenerative joint diseases and other inflammatory rheumatic diseases.1 The medical activity of S-ibuprofen is stronger than that of the R-enantiomer, and R-ibuprofen can cause side effects or toxicity, such as gastrointestinal problems. The pure enantiomers and eutectic of ibuprofen have lower melting points than racemic ibuprofen, and therefore have a higher solubility in skin lipids and a greater percutaneous absorption. It is necessary to get pure S-ibuprofen for efficient and effective medical application.2 Furthermore, many active pharmaceutical ingredients are also optically active, and in many cases, only one of the two enantiomers is pharmaceutically active. The different stereochemistry of chiral drugs between both drug enantiomers can lead to significant biochemical differences in their metabolism and efficacy.3 It has been widely recognized as one of the most difficult technical problems in organic chemistry to separate optical isomers directly. Because of regulatory requirements and considerations of therapeutic effect improvement, the market for pure enantiomers of chiral drugs is growing.4Currently, the ways to obtain single-enantiomer drugs include chiral synthesis and racemic mixture resolution.5 Racemic resolution has attracted more attention recently, including the use of chiral liquid membranes, chiral column separation, capillary electrophoresis etc. Chiral column separation and capillary electrophoresis methods have high separation ability and can be widely used and scaled up successfully, but the high cost and low yield obstruct the application of both methods. The energy requirement and separation performance of chiral liquid membranes are often reasonable.6 Pickering et al. studied the selective extraction of phenylalanine enantiomers, using copper(II) N-decyl-(L)-hydroxyproline for chiral resolution in a chiral emulsion liquid membrane process.7 The membrane phase was pre-equilibrated with an equal volume of 5 mM copper nitrate in acetate buffer at pH = 5.8. Krieg et al. used both single and multiple bulk liquid membranes (BLMs), containing β-cyclodextrin as a chiral mobile carrier, for the chiral enrichment of racemic chlorthalidone.8 Chiral enrichment was feasible, and the highest selectivity (1.41) was obtained with the multiple BLM at low pH and relative carrier concentration.
Compared with emulsion and bulk liquid membranes, supported liquid membrane could save the consumption of chiral resolution to save processing costs. A great deal of research has been conducted over several decades.9 Shinbo et al. investigated the effect of membrane solvent on transport efficiency and membrane stability in a crown ether-mediated enantioselective amino acid transport system.10 The membrane stability was assessed by operating the membranes up to 90 days. It is found that the membrane solvent must have both a high dielectric constant and low solubility in water to make the supported liquid membrane highly stable and permeable. Hadik et al. studied D,L-lactic acid and D,L-alanine solute resolution in supported liquid membrane with polypropylene hollow fiber module.11 N-3,5-dinitrobenzoyl-L-alanine-octylester, dissolved in toluene, was used as chiral resolution. The maximum D,L-lactic acid separation factor was 2.0, and that for D,L-alanine was 1.75. In both cases, the D-enantiomer flux dominated. Dzygiel et al. studied the transport of aromatic amino acids in supported liquid membrane with chiral phosphate as the carrier.12 The enantioselectivity of the process was moderate and dependent on the structure of the carrier. Clark et al. studied the resolution of a racemic mixture of phenylalanine and methionine in supported liquid membrane.13 The chiral carrier and transition metal were N-decyl-(L)-hydroxyproline and copper(II) respectively. The ratio of enantioselective equilibrium constants was determined based on initial experimental separation factors. The highest separation factors for phenylalanine and methionine were 1.8 and 1.9, respectively. Viegas et al. reported a study on the chiral resolution of propranolol with β-blockers. Propranolol was selected due to the distinct properties of its enantiomers among all β-blockers.14 Extraction and stripping kinetic studies were performed in supported liquid membrane.
In this paper, a chiral liquid membrane using L-dipentyl tartrate dissolved in octane as membrane phase was investigated to separate racemic ibuprofen. The membrane material was polyvinylidene fluoride (PVDF) hollow fiber. The feasibility of the process was studied at first. Then, the effects of chiral liquid membrane phase composition, flow rates on two sides and pH in stripping phase were investigated to determine the optimal experiment conditions. Cascade experiment was conducted to improve the optical purity of ibuprofen enantiomer.
2. Materials and methods
2.1 Reagents
Ibuprofen racemic mixture was obtained from TSKF, Tianjin, with a purity of over 99%. L-tartaric acid was obtained from GuangFu Fine Chemical, Tianjin, China. p-Toluenesulfonic acid was obtained from YiLi Fine Chemical, Beijing, China. Other reagents, including n-hexane, octane, n-octyl alcohol, and toluene, were obtained from Beijing Chemical Works, Beijing, China. All of the above reagents were of analytical grade.The L(D)-tartaric acid derivatives used in the chiral liquid membrane process were obtained by esterification between L(D)-tartaric acid and the relevant alcohol with p-toluenesulfonic acid as catalyst in our laboratory. Toluene was used to dissolve the relevant alcohol. The reaction temperature was 140 °C for the removal of formed water. After cooling down to room temperature, reaction products were washed several times with saturated sodium bicarbonate solutions and distilled water for p-toluenesulfonic acid removal. With drying to remove moisture and vacuum distillation to remove toluene, L(D)-tartaric acid ester was obtained. Structures of final products (L-dipentyl tartaric esters) were characterized by nuclear magnetic resonance (NMR) spectroscopy and Fourier transform infrared spectroscopy.
2.2 Chiral liquid membrane process
Polyvinylidene fluoride (PVDF) hollow fiber was used as the membrane material. Additional information about the hollow fiber module is listed in Table 1.| Shell characteristics | |
|---|---|
| Material | Glass |
| Length, L/m | 0.350 |
| Internal diameter, di/m | 0.016 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Fiber characteristics | |
| Material | PVDF |
| Number of fibers, N | 98 |
| Effective length, L/m | 0.300 |
| Internal diameter, dint/m | 0.000610 |
| External diameter, dext/m | 0.000872 |
| Fill factor | 0.291 |
The chiral liquid membrane was prepared at room temperature by impregnating a porous film with chiral membrane solution for at least 40 min in order to make the solution fully filled in the pores within the fibers. The hollow fiber module was operated in a recycling mode, and a schematic of the process is shown in Fig. 1.
L-tartrate ester dissolved in n-octane was used as membrane phase solution. Ibuprofen dissolved in sodium phosphate–phosphoric acid buffer solution flowed through the tube side. Sodium phosphate–phosphoric acid buffer solution flowed through the shell side. The concentration of hydroxypropyl-beta-cyclodextrin was 0.1 mol L−1. Samples were filtered with a 0.45 μm water system filtering header. All chiral liquid membrane experiments were carried out three times to ensure enough accuracy.
For the chiral liquid membrane process, the mass transfer fluxes of ibuprofen in both feed and stripping phase, Jf and Js, were determined by the following equations:15
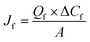 | (1) |
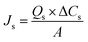 | (2) |
The separation factor (α) was calculated as:
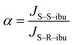 | (3) |
2.3 Analytical methods
The concentration of both ibuprofen enantiomers in aqueous phase was determined by HPLC using a UV detector (SPD-20A, Shimadzu, Japan) at a wavelength of 220 nm. A Chiral-AGP column (100 × 4.6 mm × 5.0 μm) and guard column (10 × 4.0 mm × 5.0 μm) from DAICEL (Japan) were used. The mobile phase was 0.1 mol L−1 sodium phosphate buffer solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol with a volume ratio of 98
methanol with a volume ratio of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at a flow rate of 0.5 mL min−1. Injection volume was 20 μL. The relative retention time of R-ibuprofen was about 8.4 min and of S-ibuprofen was about 11.2 min. pH values in aqueous phases were measured with a pH meter (Shanghai Dapu Instruments Co. Ltd, PXS-450, China).
2 at a flow rate of 0.5 mL min−1. Injection volume was 20 μL. The relative retention time of R-ibuprofen was about 8.4 min and of S-ibuprofen was about 11.2 min. pH values in aqueous phases were measured with a pH meter (Shanghai Dapu Instruments Co. Ltd, PXS-450, China).
3. Results and discussion
3.1 Feasibility study of chiral liquid membrane
In the feasibility study of chiral liquid membrane, hydrophobic polypropylene and PVDF hollow fibers were used as membrane materials. The membrane phase solution was L-dipentyl tartrate ester with relevant organic solution, n-octyl alcohol, n-octane and dichloroethane, separately. Membrane leakage was observed when polypropylene was used as membrane support and n-octane was used as organic solution. This might be attributed to the hydroxyl of L-dipentyl tartrate ester modifying the hydrophobicity of polypropylene membrane material. The solution in feed phase could migrate across the hollow fiber membranes to stripping phase. As a result, PVDF hollow fibers were used as membrane support in the chiral liquid membrane process. When L-tartrate ester dissolved in n-octyl alcohol or dichloroethane was used as membrane phase, both R-ibuprofen and S-ibuprofen were accumulated in the membrane phase significantly. The concentration of both ibuprofen enantiomers was low in the feed and stripping phases. This was mainly because both R-ibuprofen and S-ibuprofen preferred to dissolve in n-octyl alcohol and dichloroethane, which led to the concentration of both ibuprofen enantiomers in aqueous phase being low. So L-dipentyl tartrate ester dissolved in n-octane was used as membrane phase solution. Ibuprofen dissolved in sodium phosphate–phosphoric acid buffer solution at pH = 7.0 flowed through the tube side, while sodium phosphate–phosphoric acid buffer solution at pH = 5.0 flowed through the shell side. The flow velocities on tube and shell side were 0.352 cm s−1 and 0.210 cm s−1, respectively. Experimental results in Fig. 2 show the changes of ibuprofen enantiomer concentration with time in feed phase and stripping phase.In the feed phase, the concentration of R-ibuprofen was at the same level as S-ibuprofen. Combined with our preliminary study, the mass transfer of both ibuprofen enantiomers from feed phase to membrane phase was almost equal.16 But the concentration of S-ibuprofen was apparently higher than that of R-ibuprofen in the stripping phase. This was mainly because the stability of the complexes formed by both ibuprofen enantiomers and L-dipentyl tartrate ester was different, which led to different mass transfer rate between R-ibuprofen and S-ibuprofen. The complex formed by R-ibuprofen and L-dipentyl tartrate ester was more stable in the membrane phase, i.e. more R-ibuprofen was accumulated in the chiral liquid membrane phase. At the same time, the complexes formed by S-ibuprofen and L-dipentyl tartrate ester were transferred from membrane phase to stripping phase. As a result, more S-ibuprofen could be detected in the stripping phase. In other words, S-ibuprofen was enriched in the stripping phase by the chiral liquid membrane process. The separation factor of S-ibuprofen in the process was 1.18.
3.2 Effect of chiral liquid membrane phase composition
The kind and concentration of chiral extractants with organic solutions had an apparent effect on experimental results.15 Thus, several experiments were conducted to investigate the influence of chiral liquid membrane phase composition. | ||
| Fig. 3 The concentration of ibuprofen enantiomers in stripping phase with and without chiral extractant in membrane phase. | ||
Concentrations of R-ibuprofen and S-ibuprofen remained constant at 11 h when pure n-octane was used as the membrane phase. n-Octane showed no selectivity to both ibuprofen enantiomers, as the concentration of R-ibuprofen and S-ibuprofen remained at the same level throughout the experiment. This was mainly because there was no interaction of chiral recognition between n-octane and L-dipentyl tartrate ester. The results demonstrated that there was an interaction of chiral recognition between L-dipentyl tartrate ester and R-ibuprofen. After 8 h, the mass ratio of R-ibuprofen and S-ibuprofen in feed phase to those in stripping phase could reach 85.9% and 88.4% with chiral extractant, respectively; those values without chiral extractant were much higher, being 96.5% and 96.7%, respectively. In other words, without chiral extractant, there was almost no accumulation of ibuprofen in the membrane phase.
L-dipentyl tartrate ester and D-dipentyl tartrate ester dissolved in n-octane (0.20 mol L−1) were used as membrane phase solution. As shown in Fig. 4, the concentration of ibuprofen enantiomers in the feed phase showed the same trend with different types of chiral extractants in the membrane phase. However, the concentration of R-ibuprofen in the stripping phase is higher than that of S-ibuprofen when D-dipentyl tartrate ester was used in the membrane phase. The opposite tendency was observed for L-dipentyl tartrate ester because the interaction between S-ibuprofen and D-dipentyl tartrate ester is stronger than that between R-ibuprofen and D-dipentyl tartrate ester. However, L-dipentyl tartrate ester showed different chiral recognition characters, which led to the accumulation of R-ibuprofen in the chiral membrane phase. With D-dipentyl tartrate ester as the chiral extractant, the separation factor is 1.08 in 11 h. The results were much lower than that with L-dipentyl tartrate ester (1.18). This was mainly because the stability of the diastereomer formed with L-dipentyl tartrate ester was much greater than that formed with D-dipentyl tartrate ester.
 | ||
| Fig. 5 Influence of concentration of L-dipentyl tartrate ester in the membrane phase on the concentration of R-ibuprofen in stripping phase. | ||
 | ||
| Fig. 6 Influence of concentration of L-dipentyl tartrate in the membrane phase on the concentration of S-ibuprofen in stripping phase. | ||
The concentration of R-ibuprofen and S-ibuprofen in the stripping phase increased with the concentration increase of L-dipentyl tartrate ester in the membrane phase. This was mainly because more chiral extractant was dissolved in the membrane phase with the increase of the concentration of L-dipentyl tartrate ester. As a result, the diastereomers formed by L-dipentyl tartrate ester and both ibuprofen enantiomers were increased, and more ibuprofen enantiomers were transferred into the stripping phase.
The flux of ibuprofen in the stripping phase increased with an increase of L-dipentyl tartrate ester concentration as shown in Fig. 7. The separation factor was the highest when the concentration of chiral extractant was 0.20 mol L−1. When the concentration of chiral extractant was over 0.20 mol L−1, the flux of ibuprofen in the stripping phase remained the same while the separation factor stayed at the same level. This was mainly because the stabilities of the diastereomers formed by L-dipentyl tartrate ester and ibuprofen enantiomers were different. Ibuprofen enantiomers were selectively extracted to the organic phase with an increase of L-dipentyl tartaric ester concentration. When the concentration of L-tartaric ester was low, R-ibuprofen can be preferentially extracted from the organic phase due to competitive extraction. The result showed that the two ibuprofen enantiomers can be separated. However, with an increase of L-dipentyl tartaric ester concentration, the two ibuprofen enantiomers were extracted to the organic phase equally. The performance of competitive extraction disappeared, which resulted in the consistent separation factor when the concentration of chiral extractant was over 0.20 mol L−1. So 0.20 mol L−1 was chosen as the favorable L-dipentyl tartaric ester concentration in this research.
3.3 Effect of flow rates in two sides
The flow rates have a significant impact on the contact time and the thickness of the liquid membrane layer. In the chiral liquid membrane process, the flow rates also have an impact on the mass transfer rate of the diastereomers formed by L-dipentyl tartaric ester and ibuprofen enantiomers. Several flow rates in two sides were investigated to study the mechanism of mass transfer. Ibuprofen dissolved in sodium phosphate–phosphoric acid buffer solution flowed through the tube side at pH 7.0. Sodium phosphate–phosphoric acid buffer solution flowed through the shell side at pH 2.5. The concentration of hydroxypropyl-beta-cyclodextrin is 0.1 mol L−1. The flow rates in the tube side were 0.352 cm s−1, 0.704 cm s−1, 1.056 cm s−1 and 1.408 cm s−1, while the flow rates in the shell side were 0.210 cm s−1, 0.420 cm s−1, 0.630 cm s−1 and 0.840 cm s−1, respectively. The results are shown in Fig. 8 and 9.With an increase of flow rates in the two sides, no significant change of the mass transfer flux in stripping phase was observed. The same trend is obtained in feed phase. The experimental mass transfer resistances of ibuprofen enantiomers in both feed phase and stripping phase were not major factors. In other words, the major resistance of the process was in the chiral liquid membrane phase. This was mainly because the diastereomers formed by L-dipentyl tartaric ester and ibuprofen enantiomers were preferentially dissolved in organic phase, not in aqueous phase. The interaction of chiral recognition formed by hydrogen bonds was stable, which made ibuprofen enantiomers accumulate in the chiral liquid membrane phase.
3.4 Effect of pH in stripping phase
Ibuprofen molecule mainly exists at low pH, while ibuprofen carboxylic acid ions mainly emerge at high pH. The chiral recognition could be achieved only between ibuprofen molecules and L-tartaric ester. In this experiment, pH values in stripping phase were tested at 2.5, 4.0, 5.5 and 7.0. The influences of pH in stripping phase on the concentration of S-ibuprofen and R-ibuprofen are shown in ESI (Fig. S1 and S2†). The mass transfer flux in stripping phase and separation factor in stripping phase are shown in Fig. 10. | ||
| Fig. 10 Influence of pH in stripping phase on mass transfer flux in stripping phase and separation factor in chiral liquid membrane process. | ||
The concentrations of both R-ibuprofen and S-ibuprofen in the stripping phase increased with an increase of pH value. This was mainly because the concentration of diastereomeric compounds, which were formed by L-tartaric ester and ibuprofen enantiomers, decreased with an increase of pH value. In Fig. 10, the separation factor decreased with an increase of pH value. With an increase of pH value, the chiral recognition ability of L-tartaric ester decreased. The mass transfer rates of S-ibuprofen and R-ibuprofen were getting closer, which led to the decrease of separation factor. The mass transfer flux in the stripping phase showed the opposite trend to that of the separation factor. More S-ibuprofen and R-ibuprofen molecules were dissolved in the membrane phase, which would increase the distribution coefficient in the stripping phase. As a result, fluxes of both ibuprofen enantiomers in the stripping phase increased.
3.5 Transport results
To improve the optical purity of ibuprofen enantiomer, series operation is a common way to promote the separation performance. Although series operation needs lots of membrane modules and space, the cascade experiment used in this research needs only one membrane module. After every experiment, the membrane module was washed by deionized water thoroughly and dried by an air pump. Then the membrane module was pretreated as before. The feed and stripping phases were introduced from previous experiment. Results are shown in Fig. 11.The best separation factor was 1.38 after a six-level experiment. When the level of cascade experiment increased from 1 to 4, separation factor of ibuprofen enantiomers in the stripping phase also increased accordingly. This was because the new chiral liquid membrane phase at every level can separate ibuprofen enantiomers apart. After a 4-level cascade experiment, separation factor of ibuprofen enantiomers kept steady.
4. Conclusion
Chiral liquid membrane, using L-tartaric ester dissolved in n-octane as membrane phase, was investigated for separating ibuprofen enantiomers. S-ibuprofen was enriched in the stripping phase with the chiral liquid membrane. Polyvinylidene fluoride hollow fibers were used as membrane support in the chiral liquid membrane process. With L-dipentyl tartrate ester, the concentration of S-ibuprofen was apparently higher than that of R-ibuprofen in the stripping phase, while that with D-dipentyl tartrate ester showed the opposite trend. With D-dipentyl tartrate ester, the separation factor was 1.08, which was much lower than that with L-dipentyl tartrate ester (1.18). The optimal concentration of chiral extractant was 0.20 mol L−1. With an increase of flow rates on two sides, no significant change of the mass transfer flux in both feed and stripping phases was observed. The concentration of both R-ibuprofen and S-ibuprofen in the stripping phase increased with an increase of pH value. The separation factor decreased with an increase of pH value. The separation factor was 1.38 in cascade experiment.Acknowledgements
This work was supported by the National Natural Science Foundation (21076011 and 21276012), Program for New Century Excellent Talents in University (NCET-10-0210), and National Science & Technology Major Project (2013ZX09201006001, 2013ZX09202005 and 2014ZX09201001-006-003). The authors gratefully acknowledge these grants.References
- (a) J. D. Bradley, K. D. Brandt, B. P. Katz, L. A. Kalasinski and S. I. Ryan, N. Engl. J. Med., 1991, 325, 87–91 CrossRef CAS PubMed; (b) G. Ciabattoni, G. A. Cinotti, A. Pierucci, B. M. Simonetti, M. Manzi, F. Pugliese, P. Barsotti, G. Pecci, F. Taggi and C. Patrono, N. Engl. J. Med., 1984, 310, 279–283 CrossRef CAS PubMed; (c) S. S. Adams, R. G. Bough, E. E. Cliffe, B. Lessel and R. F. Mills, Toxicol. Appl. Pharmacol., 1969, 15, 310–330 CrossRef CAS; (d) S. S. Adams, P. Bresloff and C. G. Mason, J. Pharm. Pharmacol., 1976, 28, 256–257 CrossRef CAS PubMed; (e) E. J. Lee, K. Williams, R. Day, G. Graham and D. Champion, Br. J. Clin. Pharmacol., 1985, 19, 669–674 CrossRef CAS PubMed.
- (a) H. Shindo and J. Caldwell, Chirality, 1991, 3, 91–93 CrossRef PubMed; (b) B. Waldeck, Chirality, 1993, 5, 350–355 CrossRef CAS PubMed; (c) R. L. Nation, Clin. Pharmacokinet., 1994, 27, 249–255 CrossRef CAS PubMed; (d) S. M. Al-Saidan, J. Controlled Release, 2004, 100, 199–209 CrossRef CAS PubMed; (e) D. A. Xiong, Y. L. An, Z. Li, R. J. Ma, Y. Liu, C. L. Wu, L. Zou, L. Q. Shi and J. H. Zhang, Macromol. Rapid Commun., 2008, 29, 1895–1901 CrossRef CAS PubMed; (f) M. W. Konstan, P. J. Byard, C. L. Hoppel and P. B. Davis, N. Engl. J. Med., 1995, 332, 848–854 CrossRef CAS PubMed; (g) G. R. Bernard, A. P. Wheeler, J. A. Russell, R. Schein, W. R. Summer, K. P. Steinberg, W. J. Fulkerson, P. E. Wright, B. W. Christman, W. D. Dupont, S. B. Higgins and B. B. Swindell, N. Engl. J. Med., 1997, 336, 912–918 CrossRef CAS PubMed.
- (a) N. M. Davies, Clin. Pharmacokinet., 1998, 34, 101–154 CrossRef CAS PubMed; (b) N. M. Maier, P. Franco and W. Lindner, J. Chromatogr. A, 2001, 906, 3–33 CrossRef CAS.
- (a) N. H. Hashim and S. J. Khan, J. Chromatogr. A, 2011, 1218, 4746–4754 CrossRef CAS PubMed; (b) I. B. Rietveld, M. Barrio, B. Do, J. L. Tamarit and R. Ceolin, J. Phys. Chem. B, 2012, 116, 5568–5574 CrossRef CAS PubMed; (c) M. A. Zayed, M. F. Hawash, M. A. Fahmey and A. M. M. El-Gizouli, J. Therm. Anal. Calorim., 2012, 108, 315–322 CrossRef CAS; (d) F. Huang, B. Wang and Y. Sun, Polymer, 2013, 37, 288–293 Search PubMed; (e) X. Yuan and A. C. Capomacchia, J. Pharm. Sci., 2013, 102, 1957–1969 CrossRef CAS PubMed.
- (a) Y. H. Lu, X. J. Wang and C. B. Ching, Ind. Eng. Chem. Res., 2009, 48, 7266–7275 CrossRef CAS; (b) N. AL-Hadithi, B. Saad and M. Grote, Microchim. Acta, 2011, 172, 31–37 CrossRef CAS; (c) K. W. Tang, P. L. Zhang, C. Y. Pan and H. J. Li, AIChE J., 2011, 57, 3027–3036 CrossRef CAS PubMed.
- (a) W. R. Bowen and R. R. Nigmatullin, Sep. Sci. Technol., 2002, 37, 3227–3244 CrossRef CAS; (b) P. Hadik, L.-P. Szabó and E. Nagy, Desalination, 2002, 148, 193–198 CrossRef CAS; (c) D. Stella, J. A. Calzado, A. M. Girelli, S. Canepari, R. Bucci, C. Palet and M. Valiente, J. Sep. Sci., 2002, 25, 229–238 CrossRef CAS; (d) N. H. Hashim, L. D. Nghiem, R. M. Stuetz and S. J. Khan, Water Res., 2011, 45, 6249–6258 CrossRef CAS PubMed.
- (a) P. J. Pickering and J. B. Chaudhuri, Chirality, 1997, 9, 261–267 CrossRef CAS; (b) P. J. Pickering and J. B. Chaudhuri, J. Membr. Sci., 1997, 127, 115–130 CrossRef CAS.
- H. M. Krieg, J. Lotter, K. Keizer and J. C. Breytenbach, J. Membr. Sci., 2000, 167, 33–45 CrossRef CAS.
- (a) E. Miyako, T. Maruyama, N. Kamiya and M. Goto, Chem. Commun., 2003, 2926–2927 RSC; (b) F. P. Jiao, K. L. Huang, X. H. Peng, X. H. Zhao and J. G. Yu, J. Cent. South Univ. Technol., 2006, 13, 39–43 CrossRef CAS PubMed; (c) A. Maximini, H. Chmiel, H. Holdik and N. W. Maier, J. Membr. Sci., 2006, 276, 221–231 CrossRef CAS PubMed; (d) H. Meng, S. M. Li, L. Xiao and C. X. Li, AIChE J., 2013, 59, 4772–4779 CrossRef CAS PubMed.
- T. Shinbo, T. Yamaguchi, H. Yanagishita, K. Sakaki, D. Kitamoto and M. Sugiura, J. Membr. Sci., 1993, 84, 241–248 CrossRef CAS.
- P. Hadik, L. Kotsis, M. Eniszné-Bódogh, L.-P. Szabó and E. Nagy, Sep. Purif. Technol., 2005, 41, 299–304 CrossRef CAS PubMed.
- (a) P. Dźygiel, P. Wieczorek, J. K. Jonsson, M. Milewska and P. Kafarski, Tetrahedron, 1999, 55, 9923–9932 CrossRef; (b) P. Dzygiel, P. Wieczorek and P. Kafarski, J. Sep. Sci., 2003, 26, 1050–1056 CrossRef CAS PubMed.
- J. D. Clark, B. B. Han, A. S. Bhown and S. R. Wickramasinghe, Sep. Purif. Technol., 2005, 42, 201–211 CrossRef CAS PubMed.
- R. M. C. Viegas, C. A. M. Afonso, J. G. Crespo and I. M. Coelhoso, J. Membr. Sci., 2007, 305, 203–214 CrossRef CAS PubMed.
- R. B. Bird, W. E. Stewart and E. N. Lightfoot, in Transport Phenomena, ed. W. Anderson, Wiley, New York, 2nd edn, 2006, partIII, ch. 17, p. 536 Search PubMed.
- Z. Ren, Y. Zeng, Y. Hua, Y. Cheng and Z. Guo, J. Chem. Eng. Data, 2014, 59, 2517–2522 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04764a |
| This journal is © The Royal Society of Chemistry 2015 |







