Cyclosporine A loaded self-nanoemulsifying drug delivery system (SNEDDS): implication of a functional excipient based co-encapsulation strategy on oral bioavailability and nephrotoxicity†‡
Abstract
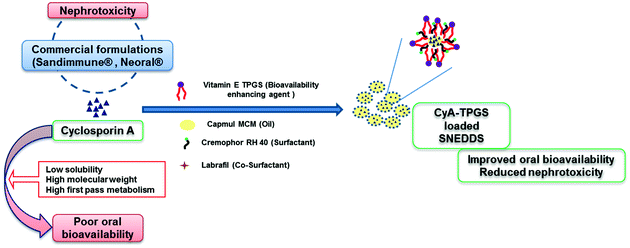
The present work focusses on the formulation development and evaluation of a functional excipient (surfactant stabilizer), a vitamin E TPGS loaded self-nanoemulsifying drug delivery system (SNEDDS), for improving the deliverability and safety profile of cyclosporine A (CyA). The saturation solubility of individual bioactive compounds were evaluated in a series of oils and surfactants. In addition, a ternary phase diagram-based exhaustive optimization was performed to identify the nanoemulsification region that yields the desired quality of attributes and maximum loading capacity of CyA and vitamin E TPGS. The optimized formulation exhibited excellent stability in simulated gastrointestinal fluids. In vitro drug release studies revealed significantly higher rapid release of CyA from CyA-TPGS SNEDDS as compared to that of the clinically available counterpart Bioral® or in-house CyA-SNEDDS. In vivo pharmacokinetics further demonstrated a 4.48-fold increase in oral bioavailability in the case of the developed formulation as compared to Bioral®. CyA induced reactive oxygen species (ROS) generation in HEK cell lines was significantly diminished in the case of CyA-TPGS SNEDDS in contrast to that of CyA SNEDDS, Bioral® and the CyA + vitamin E TPGS physical mixture. The results were further corroborated by in vivo nephrotoxicity studies wherein the levels of biochemical markers of nephrotoxicity, blood urea nitrogen and serum creatinine levels were comparable to that of a negative control in the case of the developed formulation in contrast to that of Bioral®. In a /nutshell, the employed strategy of functional excipient loaded SNEDDS poses a viable strategy for developing value-added nanoformulations of CyA.

 Please wait while we load your content...
Please wait while we load your content...