Monodisperse erythrocyte-sized and acid-soluble chitosan microspheres prepared via electrospraying
Abstract
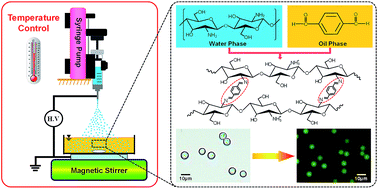
Monodisperse erythrocyte-sized and acid-soluble chitosan microspheres are successfully prepared by an electrospraying method for the first time. Effects of the physical properties of the polymer solution and the condition parameters of the electrospraying process on the size and size distribution of the chitosan microspheres are systematically studied, to optimize the conditions for preparation of chitosan microspheres with our specific requirements. The microsphere size is mainly controlled by the viscosity of the spray liquid, and the microsphere monodispersity mainly depends on the electric conductivity of the spray liquid, the flow rate and the needle size. Under the optimized conditions, monodisperse chitosan microspheres with an average diameter of 6.4 μm, which is similar to the erythrocyte size, are prepared with a narrow size distribution (CV < 3%). Due to the use of terephthalaldehyde as cross-linker via formation of Schiff base bonds, the prepared chitosan microspheres can maintain structural integrity and show green fluorescence in a neutral medium, but display rapid acid-triggered decomposition. The prepared erythrocyte-sized and acid-soluble chitosan microspheres are highly attractive as promising substitutes of blood samples for calibration of hematology analyzers and flow cytometers.

 Please wait while we load your content...
Please wait while we load your content...