Hierarchical polymorphic MnCO3 series induced by cobalt doping via a one-pot hydrothermal route for CO catalytic oxidation†
Abstract
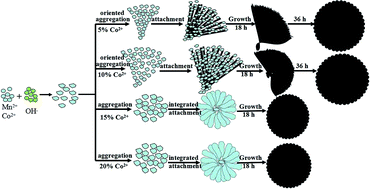
In this study, a series of cobalt-doped MnCO3 hierarchical microstructures with different morphologies were synthesized by tuning a single variable (the dopant content) via a one-step, mild solvothermal synthesis in a N,N-dimethylformamide (DMF) solution system. Structure evolution of the polymorphic MnCO3 took place with the morphology obviously transforming from an initial flower-microstructure to fan-like, then to hedgehog-like hemispheres and finally to flake-spheres as the Co2+ theoretical content (Co/(Co + Mn)) increased from 0 to 20% in the solvothermal process. Cobalt ion modulated reaction-limited aggregation (RLA) is proposed in the growth mechanism. The mechanism of Co2+-induced acceleration and full growth is further investigated. The Co2+ doped manganese carbonate displays wonderful catalytic performance towards CO oxidation.

 Please wait while we load your content...
Please wait while we load your content...