A novel high performance oxazine derivative: design of tetrafunctional monomer, step-wise ring-opening polymerization, improved thermal property and broadened processing window
Abstract
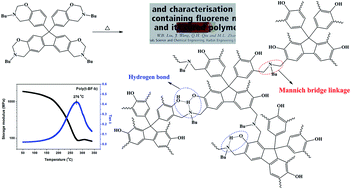
A novel tetraphenol fluorene, 2,7-dihydroxy-9,9-bis-(4-hydroxyphenyl)fluorene (THPF), was synthesized via the condensation reaction of 2,7-dihydroxy-9-fluorenone and phenol in the presence of a strong acidic cation exchange resin and 3-mercaptopropionic acid. Thus, a novel tetrafunctional oxazine monomer containing benzoxazine and fluorene-oxazine (t-BF-b) was prepared for the first time using a Mannich condensation reaction of THPF with paraformaldehyde and n-butylamine. The chemical structures of THPF and t-BF-b were characterized by Fourier transform infrared (FTIR) spectroscopy, elemental analysis, 1H and 13C nuclear magnetic resonance (NMR). The viscosity–temperature properties and the polymerization behavior of t-BF-b as well as the thermal and mechanical properties of its cured polymer (poly(t-BF-b)) were studied by rheometry, FTIR, 1H NMR, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and dynamic mechanical analysis (DMA). The results show that poly(t-BF-b) displays a lower melting point and wider processing window. The oxazine rings of fluorene-oxazine possess higher reactivity and lower polymerization temperature than those of benzoxazine. Also, its cured poly(t-BF-b) exhibits a higher glass transition temperature than its corresponding bifunctional polybenzoxazines without sacrificing any thermal properties in spite of the introduction of more flexible aliphatic groups into polymer chains.

 Please wait while we load your content...
Please wait while we load your content...