Solid acid-reduced graphene oxide nanohybrid for enhancing thermal stability, mechanical property and flame retardancy of polypropylene†
Abstract
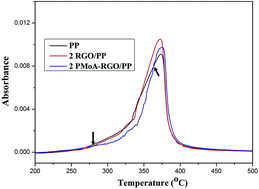
Reduced graphene oxide (RGO) is functionalized with a solid acid, phosphomolybdic acid (PMoA), via electrostatic interactions. RGO and PMoA in this nanohybrid (PMoA–RGO) exhibit strong interactions and the surface characteristic of the graphene nanosheets is modified. RGO and PMoA–RGO are blended with polypropylene (PP) and maleic anhydride grafted polypropylene via a master batch-based melt mixing method. Thermal stability, mechanical and flame retardancy properties of the nanocomposites are investigated. This nanohybrid greatly improves the stiffness and thermal-oxidative stability of PP. Compared to the neat sample, the onset decomposition temperature (Tonset) and the temperature at the maximum weight loss rate (Tmax) of the nanocomposite increase by as much as 44 °C and 34 °C, respectively, at just 1 wt% loading of PMoA–RGO. Remarkable enhancements of the storage modulus in the glassy region and heat deflection temperature are obtained in PMoA–RGO/PP nanocomposites. The nanohybrid exhibits more marked reinforcing effects than the RGO. The heat release of the nanocomposites during the combustion is considerably reduced compared to neat PP. The improved thermal-oxidative stability and flame retardant properties of PP nanocomposites are mainly attributed to the barrier effect of graphene, in tandem with the enhanced radical trapping property of the nanohybrid.

 Please wait while we load your content...
Please wait while we load your content...