MFC with vermicompost soil: power generation with additional importance of waste management
Abstract
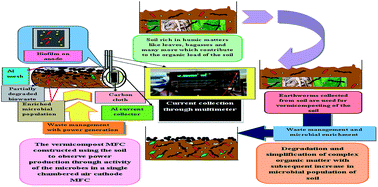
In the present study, vermicomposted soil has been analyzed as a substrate feed in a microbial fuel cell (MFC) for harvesting bioenergy. The results have shown that composting aided in providing a better environment with available organic matter and enriched microbial population. A maximum 66% COD removal with a highest power density of 4 mW m−2 has been observed from a MFC with vermicomposted soil. In comparison, a maximum power density of 134.44 μW m−2 with 31% COD removal has been observed for a MFC with non-vermicomposted (control) soil. The differences were mainly due to the predominant microbial species and organic matter additionally available in vermicomposted soil. In cyclic voltammetric analysis, the microbes present in the vermicomposted soil were found to be electrogenic. Electrochemical Impedence Spectroscopy (EIS) analysis allowed the evaluation of the internal resistance of the single chambered cell. Scanning electron microscopy (SEM) images revealed microbial association with the process, whilst energy-dispersive X-ray spectroscopy (EDS) analysis provided results for the elemental analysis of both kinds of soil.

 Please wait while we load your content...
Please wait while we load your content...