An in situ crosslinking approach towards chitosan-based semi-IPN hybrid particles for versatile adsorptions of toxins†
Abstract
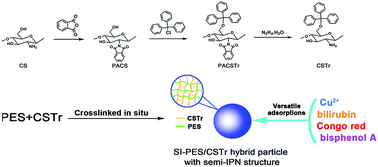
The application of chitosan (CS) as adsorbent was limited by its poor acidic resistance, solubility in organic solvents and miscibility with synthetic polymers. In this study, an effective way was utilized to increase the amount of CS in hybrid particles together with good acid–alkali resistance and mechanical strength. CS was chemically modified and in situ cross-linked in polymer solutions to prepare hybrid particles with a semi-interpenetrating network (semi-IPN) structure. Polyethersulfone (PES) was chosen as a representative polymer matrix to prepare hybrid particles; and the PES/CS-derivative particles exhibited good adsorption capacity to the toxin bilirubin without procoagulant activity and adsorption capacity to Cu2+. Furthermore, the PES/CS-derivative hybrid particles exhibited selective adsorption to the anionic dye Congo Red (CR) and the environmental hormone bisphenol A (BPA) compared with other dyes or phenols. The results indicated the hybrid particles had versatile applications in blood purification and wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...