Characterization of malic enzyme and the regulation of its activity and metabolic engineering on lipid production
Ying-Jie Lianga and
Jian-Guo Jiang*ab
aSchool of Biological Science & Engineering, South China University of Technology, Guangzhou, 510006, China. E-mail: jgjiang@scut.edu.cn; Fax: +86-20-87113849; Tel: +86-20-87113849
bCollege of Food Science and Engineering, South China University of Technology, Guangzhou, 510640, China
First published on 14th May 2015
Abstract
Nowadays, microbial lipids are employed as the feedstock for biodiesel production, which has attracted great attention across the whole world. Malic enzyme (ME) is a key enzyme regulating the lipid accumulation process in oleaginous microorganisms. It catalyzes the oxidative decarboxylation of L-malate to pyruvate and CO2 with concomitant reduction of NADP+ to NADPH, supplying the reducing power for fatty acid biosynthesis. The extent of lipid accumulation in some fungi is identified to be controlled by ME acting as the sole source of NADPH. This review covers related research about molecular characterization and biochemical properties of MEs from various sources, and summarizes several possible modulators that affect ME activity during the lipid production process. If those harmful effects on ME activity throughout the lipid accumulation can be eliminated, more lipids can be produced. In addition, recent progress in overexpression of the ME gene for lipid biosynthesis is discussed. Quite a few successful stories in lipid overproduction by homologous or heterogenous overexpression of ME have occurred in some transformed microbial strains, indicating that ME is a promising target for gene transformation. However, the role of ME in the regulation of lipid biosynthesis is challenging in some cases.
Introduction
Microbial lipid and its biosynthesis
Oleaginous microorganisms refer to microorganisms with a lipid content in excess of 20% biomass weight.1 Microbial lipid, also named as single cell oil (SCO), has become an economic reality and has attracted great attention all over the world.2 SCO is rich in polyunsaturated fatty acids (PUFAs), such as γ-linolenic acid (GLA), eicosapentaenoic acid, arachidonic acid (ARA; 20:4 n-6) and docosahexaenoic acid (DHA; 22:6 n-3), which are commercially utilized in infant formulae, food, nutritional and pharmaceutical industries.3–5 On the other hand, high oil prices, environmental problems and a petroleum supply imbalance have spurred the search for renewable energy sources. Microbial production of oils can be converted to biodiesel, becoming a promising alternative to fossil fuels.6,7 Further, in comparison with plant oils and animal fats, microbial lipids have many advantages, such as short life cycle, ample and cheap feedstock, less labor needed, less influence by place, season and climate, and easier to scale up.8,9Most of the lipid-producing microorganisms such as microalgae, bacteria, fungi and yeast are potential oil feedstocks for biodiesel production.1,10 To further develop the SCO process, it is important to understand how microbes synthesize their fatty acids and why they are capable of producing so much lipid.2 Researchers have studied the biochemistry of lipid accumulation in yeasts and filamentous fungi: lipid accumulation is caused in cells when exhausting nitrogen from the nutrient medium, but glucose goes on to be absorbed.11 Microbial lipids biosynthesis process involves fatty acid synthesis approach and triacylglycerol (TAG) synthesis approach.12,13 To synthesize fatty acid, it is necessary for the microbes to have a continuous provision of acetyl-CoA as an essential precursor for fatty acid synthetase (FAS), and a sufficient supply of NADPH in the cytosol (Fig. 1).11 NADPH as an essential reductant required for fatty acid biosynthesis, is primarily produced by malic enzyme (ME).14 It is suggested that most of the oleaginous microorganisms have a concerted lipogenic metabolon complex (Fig. 2) that associates with ME, ATP: citrate lyase (ACL) and FAS to create a pathway for the metabolites (NADPH and acetyl-CoA) toward the FAS active sites. Eventually the fatty acids change into TAG and packed via the endoplasmic reticulum to become fatty acid droplets.2,15
 | ||
| Fig. 1 The cytosolic ‘transhydrogenase cycle’ and the ‘citrate-malate cycle’ can supply adequate precursors of acetyl-CoA and NADPH for lipid accumulation in most oleaginous yeast and fungi. The ‘transhydrogenase cycle’ can work independently of the carbon efflux from citrate in the mitochondrion to acetyl-CoA in the cytosol and therefore can supply the NADPH required for reactions of fatty acid biosynthesis, desaturation and elongation. ACL: ATP: citrate lyase; CMT: citrate/malate translocase; CS: citrate synthase; MDH: malate dehydrogenase; ME: malic enzyme; PDC: pyruvate decarboxylase; PDH: pyruvate dehydrogenase; PYC: pyruvate carboxylase.2 | ||
 | ||
| Fig. 2 The lipogenic metabolon complex associates with ME, ACL and FAS to create a pathway for the metabolites (NADPH and acetyl-CoA) toward the FAS active sites. Eventually the fatty acids change into TAG and are packed via the endoplasmic reticulum to become fatty acid droplets. OAA: oxaloacetate; AC-CoA: acetyl-CoA; Mal-CoA: malonyl-CoA; FAS: fatty acid synthase; ACL: ATP: citrate lyase; ACC: acetyl-CoA carboxylase; CMT: citrate/malate translocase; ME: malic enzyme; PC: pyruvate carboxylase; MDH: malate dehydrogenase.2 | ||
Malic enzyme—the key to the regulation of lipid biosynthesis
Malic enzyme (ME) is a type of oxidative decarboxylases which have been characterized from different sources.16 It is a well-known enzyme implicated in several biological functions in vivo and participates in various metabolic pathways such as photosynthesis, lipogenesis, energy metabolism and anaerobic growth.17–20 During lipogenesis process, two molecules of NADPH are needed for elongation of the fatty acid acyl chain.21 And NADPH is considered to be supplied by ME via this reaction: L-malate + NADP+ → pyruvate + CO2 + NADPH.22 A divalent metal ion is required as cofactor for the reaction.16 In view of NADPH as the requisite reducing power in lipid production, the stability of ME is therefore crucial and ME activity is significant to be studied in the regulation of lipid accumulation. It was proposed that a common pool of NADPH produced by various enzymes didn't exist in oleaginous microorganisms. Nevertheless, it is the lipid metabolon formation among ME, ACL and FAS that promote the fatty acid elongation (Fig. 2).2,23Previous reports on the two oleaginous fungi, Mucor circinelloides and Mortierella alpine, have established the unique role of ME to provide NADPH for fatty acid synthesis as well as fatty acid desaturation.23,24 It was suggested that the extent of lipid production was controlled by ME and no other NADPH-generating enzyme appeared able to supply the reducing force for FAS.2,23 The production of NADPH by ME was recognized as a rate-limiting factor when oleaginous fungi synthesized fatty acids.25 For example, lipid accumulation strongly correlated to the Mc. circinelloides ME activity which was inhibited by sesamol, implying that the deprivation of ME activity limited the lipid accumulation.26 Also a mutant Aspergillus nidulans (scuK248) without ME activity was shown to produce half the lipid of the wild-type possessing ME when growing under exactly the same conditions. However, ME did not seem to confine the formation of metabolically lipid (e.g. membrane lipids) as the cells were able to utilize NADPH from other sources.22,27 In Cunninghamella sp. 2A1, it was reported that the decrease of ME activity was consistent with the cease of lipid accumulation.4 Also in the yeast Rhodosporidium toruloides, ME was reported to serve as the sole source of NADPH for fatty acid biosynthesis.28 However, the role of ME during lipid accumulation in some oleaginous microorganisms was challenged (discussed later). This review is covering the related researches about molecular characterization, biochemical properties of ME and the regulations of its activity and genetic engineering on lipid production.
Molecular characterizations of ME
The classification and structure of ME
The activity of ME was first found in pigeon liver and was later widely discovered in prokaryotes, fungi, plants and animals.19,29 In eukaryotes, MEs occur in the cytosol or mitochondria. In the case of plants, MEs exist in the chloroplasts.30 According to the substrate specificity and coenzyme preference, MEs can be categorized into three types. The first type (NAD+-ME, EC 1.1.1.38) is NAD+-dependent and capable of decarboxylating oxaloacetic acid (OAA). The second type (NAD(P)+-ME, EC 1.1.1.39) tends to utilize NAD+ rather than NADP+ but cannot decarboxylate OAA. The third type (NADP+-ME, EC 1.1.1.40) only utilizes NADP+ and can decarboxylate OAA.31MEs from different organisms shared conserved amino acid sequences and structures. Such shared features play an important part in the biological functions of these enzymes.16,32 The human mitochondrial NAD(P)-ME (m-NAD(P)-ME) in a binary complex with NAD+ was the first crystal structure of ME. Additionally, the crystal structures of pigeon cytosolic NADP+-ME and the NAD+-ME from Ascaris suum have been recorded as well.33–35 According to their crystal structure, MEs are homotetramer with a double dimer structure in which the dimer interface facilitates stronger interaction than the tetramer interface. Besides, distinct characters of tetramer organization have been reported for ME.32 The structure information demonstrated that the special backbone structure of MEs had a diverse topology from any other oxidative decarboxylases, proving that it belonged to a new class of oxidative decarboxylases.16,36 In addition, the ME from E. coli was an octamer, whereas large animal MEs were tetramers with identical or similar subunits. ME from the Sulfolobus solfataricus was a dimer, but it presented as a monomer from the Lactobacillus casei.37 What's more, NADP-ME4 from the Arabidopsis presented in equilibrium of active dimers and tetramers, whereas the cytosolic counterparts exist in hexamers or octamers.38
Conserved domains and functional motifs in ME
Mc. circinelloides is one of the commercial lipid production species. NCBI-CD search revealed that there are two mainly domains in the amino acid of Mc. circinelloides ME. Malic superfamily is an N-terminal domain of ME and the NAD_bind_amino_acid_DH superfamily is a member of the Rossmann fold superfamily (Fig. 3). In addition, a PTA (phosphotransacetylase)-like domain, was found in the C-terminal part of the ME from R. palustris no. 7, Sh. meliloti and E. coli.39–41 However, no PTA activity was discovered in ME from R. palustris no.7 and Sh. Meliloti.39 In E. coli ME, the PTA domain was not active and not crucial for the ME reaction. However, it appeared to participate in allosteric regulation of ME.41 Furthermore, interception of the PTA domain made E. coli ME insensitive to acetyl-CoA, indicating the role of PTA domain in regulating the bond of acetyl-CoA. The phenomenon that acetyl-CoA allosterically restrained ME activity was coincided with this.39The amino acid sequences of MEs from different organisms were retrieved from the NCBI database (http://ncbi.nlm.nih.gov). The putative ME sequences were then aligned using ClustalX. Several conserved motifs were found in them (Fig. 4). GXGXXG/A (motif 2 and motif 4 in Fig. 4) were two dinucleotide-binding signature motifs. The structure demonstrated that one was related to the bond of an NAD+ molecule. The other existed in the active site and might be related to the substrate binding.42 The alleged divalent metal cation binding motif, (F/Y)ED-…-FNDD (motif 3 in Fig. 4), was discovered between the above two dinucleotide binding signature motifs. Previous study depicted its participation in the binding of divalent metal cations, for example, Mn2+and Mg2+.43,44 Recently, researchers found a distinctive insertion motif FLxxPG in the N-terminal of the NADP+-ME from Mucoromycotina (corresponded to motif 1 in Fig. 4), which was not discovered in other fungi. It was probably a portion of the dimer interface area of the enzyme, supplying a continuous positively charged vessel for the effective connecting of negatively charged effector molecules. It was indicated that this insertion motif might relate to special kinetic of the ME and the lipid accumulation of industrially important oleaginous microorganisms. However, this should be further experimentally verified.43
Isoforms of ME from Mc. circinelloides and Mt. alpina
Previously, it was proposed that ME could fulfill several roles simultaneously. Therefore it might present in multifarious isoforms coded by several genes.45 A study showed that there were at least six isoforms (I to VI) of ME (EC 1.1.1.40) in Mc. circinelloides CBS 108.16. Only isoform IV correlated to lipid accumulation, occurring only after N-depletion when cells growing in glucose. It was inferred that isoform IV was generated from isoform III via post-translational modification induced by either N-restriction with glucose as the carbon source or growth on acetate as the sole carbon source. Maybe these two isoforms were coded by the same single gene because their subunits and N-terminal amino acid sequences were identical and isoform IV occurred as soon as isoform III disappeared. Isoform III seemed to be constitutive and appeared under active (balanced) growth circumstance and was therefore important for basic metabolism. Isoforms I, II, V and VI correlated to anaerobic growth and only appeared under O2-limited circumstance.19 In another study of Mc. circinelloides, the ME was demonstrated to be influenced by dissolved oxygen tension (DOT). The expression of isoform IV was induced by high DOT level, according with lipid biosynthesis, while expression of isoform I, II, III, and V were induced by low DOT level.46A similar phenomenon was found in Mt. alpine Peyron CBS 696.70 with at least seven isoforms (A–G) of ME. Isoforms A and B in Mt. alpine might correspond to isoforms I and II in Mc. circinelloides, respectively; isoforms F and G might match isoforms V and VI. However, isoform C corresponded to nothing in Mc. circinelloides. Likewise, only isoform E, which produced from isoform D, was associated with lipid accumulation, becoming obvious when lipogenesis occurred after N-limitation.47 However, the precise mechanism that isoforms III/D converted to isoforms IV/E was not determined.48 Isoform D and E appeared under both anaerobic and aerobic growth circumstances. Other five isoforms correlated to O2-limited growth, involving fixing CO2 via the adverse reaction from pyruvate to malate. Moreover, isoform E gradually transformed to isoform G at −20 °C, indicating that modification of ME after translation occurred. Therefore an intense similarity existed between these two fungi: the expression of ME occurred during balanced growth and then lipid production. Certainly, ME should own such numbers of isoforms because of its participation in various metabolic pathways; regulation on the expression and activity of the isoforms would be essential to satisfy the demand of the cells.47
In addition, in Cunninghamella bainieri 2A1, activity of isoform E was intensely associated with the profiles and the level of lipid biosynthesis in N-limitation condition, while isoform D reduced as lipid produced. However, with ammonium tartrate as nitrogen source, activity of isoform D was pronounced, while isoform E was very low. It supported further evidence that isoform E was the crucial regulator of lipid accumulation in C. bainieri 2A1 and should be taken into account in future investigation regarding to escalation of lipid production.49
Identification and characterization of ME genes
The genes of ME have been cloned and characterized from various species. ME genes from plants were demonstrated to be multigene family, e.g., the Arabidopsis genome comprised two NAD-ME genes and four NADP-ME genes.38 It was reported that Yarrowia lipolytica contained only one ME gene and the ME existed in the mitochondrion.50,51 In Lipomyces starkeyi, the ME existed in the mitochondrion too and was possibly the sole isoform in this microbe.52 Researchers characterized the gene named mce1 coding for isoform II of ME related to anaerobic growth in Mc. circinelloides and this gene had similarity in sequence to ME gene from Saccharomyces cerevisiae mitochondrion and anaerobic fungus, Neocallimastix frontali.18,47 In the recent year, two genes had been identified in Mt. alpine.53 Five ME genes had been annotated and analysed in Mc. circinelloides with two coding for mitochondrial MEs and three coding for cytosolic ones. It was proposed that one ME gene (ID 182779) encoded the isoform III and IV of ME.48 In Pythium splendens, 11 genes coding for ME have been annotated.54Researchers found that some feasible regulators was related to hormone and light responses in the ME promoter region from Dunaliella parva, containing the ABRE (abscisic acid respond ability), CGTCA-motif (MeJA-responsiveness), ERE (ethylene-responsive element), light responsive element (BoxI, G-Box, and Sp1) and ARE (regulator crucial for the anaerobic reaction). It was indicated that the ME gene expression could be adjusted by environmental aspects like hormone and light through each of the response factors.55 A reduplicative TATA sequence and a CCAAT motif, which do not exist in house-keeping genes, appeared in the ME promoter from Mc. circinelloides. It was deduced that the ME isoforms were expressed under special physiological circumstance, each of them playing respective biochemical roles. Also, a CT rich region was discovered upstream of the translational start point, which was ordinary among fungal promoters.18
Biochemical properties of ME
Effect of divalent metal ions on ME activity
ME catalyzes the oxidative decarboxylation of L-malate to pyruvate and CO2 and simultaneously generates NAD(P)H from NAD(P)+. A divalent metal ion is required as cofactor for the reaction.16 When there was no divalent metal ion in the culture media, ME activity was just 11% of the control in Mt. alpine, and was totally abolished by 0.1 mM EDTA.45 However, different divalent metal ions have different effects. MEs from some sources were dependent on Mg2+ or Mn2+ for activity.45,56,57 Some preferred Mn2+ as a divalent cation.56 Some appeared to be activated by Co2+, Ni2+.31,45 The metal ion acted as a bridge between malate and ME to properly position the substrate at the active site center.58 In E. coli, the Mg2+ and Mn2+ seemed to steady two different conformations of ME.59Other cations such as Ca2+, Cu2+, Fe2+ and Sr2+, exhibited inhibition on the ME.31,60 The Mt. alpine ME was inhibited by Zn2+ at high concentration but slightly activated by Zn2+ at low concentrations and the analogous situation occurred in pigeon liver and Rhizobium meliloti.45,61,62 It was because that the ligancy between vital ions and the ME was six, but in Cu2+ and Zn2+ it was just four. It was tetrahedral or distorted tetrahedral in the metalloenzyme, which deactivated the ME.45
Effect of the metabolic analogues of substrate on ME activity
It was demonstrated that most intermediates of the TCA cycle except succinic acid show inhibition on the ME activity. And the structural analogues of L-malate (e.g., citrate, isocitrate, OAA and α-ketoglutaric acid) serve as the most efficient inhibiting agent of ME activity (Table 1).51,63 Fumarate as a trans dicarboxylic acid, could allosterically activate the ME from human. However, maleic acid, the cis-isomer of fumarate, was a remarkable inhibitor of ME, indicating that the trans configuration was vital for allosteric regulation of ME. Succinate was an activator of ME, however, the diamide of succinate, succinamide, inhibited the ME activity.63 Other dicarboxylic acids such as glutaconic acid, malonic acid and α-ketoglutaric acid, were inhibitors of ME.45,57,63 It was inferred that though these structural analogues might get into the allosteric site to suppress ME activity, they were chief active-site restrainer of ME. Furthermore, the trans conformation was important for dicarboxylic acid to allosterically activate the enzyme.63| Sources | ME | Inhibitor/activator | Effect | Note | References |
|---|---|---|---|---|---|
| Y. lipolytica | NAD(P)-ME | Citrate, isocitrate | − | Strongly inhibited in a dose-dependent manner | 51 |
| Human | m-NAD(P)-ME | Fumarate | + | An allosteric activator | 63 |
| Maleic acid | − | A remarkable inhibitor | |||
| Succinate | + | Weaker than fumarate | |||
| Succinamide | − | A poor active-site inhibitor | |||
| Glutaconic acid | − | An active-site inhibitor | |||
| Malonic acid | − | An active-site inhibitor | |||
| α-Ketoglutarate | − | An active-site inhibitor | |||
| Mt. alpine | NADP-ME | OAA | − | Feedback inhibition | 45 |
| Glyoxylate | − | Competitive inhibition | |||
| Mc. circinelloides | NADP-ME | Tartronic acid | − | Inhibit ME at 10 mM | 57 |
| Glutaric acid | − | Inhibit ME at 10 mM | |||
| 1-Methylenecyclopropane trans-2,3-dicarboxylic acid | − | Inhibit ME at 10 mM | |||
| R. palustris no. 7 | NAD(P)-ME | OAA | − | Competitive inhibition | 39 |
| Fructose-6-phosphate | − | Competitive inhibition | |||
| Acetyl-CoA | − | Allosteric inhibition | |||
| E. coli | MaeB | Acetyl-CoA | − | No effect on SfcA | 41 |
| Glutamate and aspartate | + | SfcA was activated only by aspartate | |||
| Glucose-6P | + | Allosteric activation | |||
| SfcA, MaeB | Acetyl-P | ± | An inhibitor of SfcA but an activator of MaeB | ||
| SfcA | CoA | − | Little effect on MaeB |
OAA and glyoxylate suppressed ME from Mt. alpina. These two metabolites had a spatial organization like L-malate so that it competed against L-malate by fitting the active site and then suppressed the ME activity.45 Further, the reaction that L-malate transformed to pyruvate catalyzed by ME could explain the inhibited effect of OAA. Firstly, L-malate was dehydrogenated to OAA with formation of NADPH. Secondly OAA was decarboxylated to pyruvate. OAA could feedback-inhibit the second step of the reaction so that the production of NADPH is reduced.16,45 Also, OAA inhibited the MEs from the E. coli, Mc. circinelloides and R. palustris no. 7.39,41,57 Some metabolic analogues of malate: tartronic acid, glutaric acid and 1-methylenecyclopropane trans-2,3-dicarboxylic acid inhibited ME activity.57 Fructose-6-phosphate competitively inhibited the activity of ME from R. palustris no. 7.39
Acetyl-CoA was an inhibitor of NADP-ME (MaeB) from E. coli and allosterically inhibited ME from R. palustris no. 7. But it had no effect on NAD(P)-ME (SfcA) from E. coli.39,41 Acetyl-P showed inhibition on SfcA but activation on MaeB. MaeB showed activation by amino acids (glutamate and aspartate) and sugar phosphates (glucose-6-phosphate), while SfcA was motivated just by aspartate. It was indicated that ME was very active with high-energy bonds or carbon-containing molecules at high concentration. Furthermore, CoA had little effect on MaeB but inhibited SfcA. Altogether, the consequences indicated that the regulation of MaeB and SfcA activities appeared to correlate to the activation of the reverse or forward PTA reaction (interconversion of acetyl-CoA and acetyl-P).41
The possible modulators of ME activity in lipid production
Given the significance of ME in the regulation of lipid biosynthesis, it is essential to figure out what control the activity of ME during the lipogenesis period. So far, the activity of ME has been demonstrated to be regulated by the control of the ME gene, feedback inhibition, ME-cleaving enzyme, malic acid, phosphate-limitation condition, metal ions, lignocellulosic hydrolysates, nitrogen source, mixed β-group vitamins and short-chain fatty acids (Table 2).| Microorganisms | Culture condition | ME activity | Note | References |
|---|---|---|---|---|
| Schizochytrium sp.HX-308 (algae) | 4 g L−1 malic acid | Increased | +15% lipid content; DHA percentage increased from 35% to 60% | 64 |
| 0.1 g L−1 KH2PO4 | 362.1 U mg−1 | 46.2% DCW of lipid content; 148.3 mg L−1 h−1 of DHA | 65 | |
| 4 g L−1 KH2PO4 | 326.3 U mg−1 | 42.8% DCW of lipid content; 86.1 mg L−1 h−1 of DHA | ||
| Cunninghamella sp. 2A1 (mold) | Mg2+ | Increased | +64% lipid content | 66 |
| Fe2+ | Increased | +43% lipid content | ||
| Zn2+ | Increased | +33% lipid content; +74% of GLA yield | ||
| A. niger A60 (mold) | 0.1 mM Cu2+ | Be abolished | −44% total lipid | 60 |
| T. fermentans (yeast) | Vanillyl alcohol (<25 mM) | Increased | +Lipid production | 67 |
| Catechol | Inhibitory | −Lipid production | ||
| Hydroquinone | Inhibitory | −Lipid production | ||
| Furfuryl alcohol | Inhibitory | −Lipid production | ||
| Furoic acid | Increased | +Lipid production | 68 | |
| Vanillic acid | Inhibitory | −Lipid production | ||
| Vanillin | Increased | −Lipid production | 69 | |
| Syringaldehyde | Decreased | −Lipid production | ||
| C. echinulata CCF-103 (mold) | NaNO3 | 44.8 (nmol min−1 mg−1) | 12.6 (%, w/w) lipid | 70 |
| Organic nitrogen (corn-steep) | 112.4 (nmol min−1 mg−1) | 33.0 (%, w/w) lipid | ||
| Mc. circinelloides | Aspartate | 30 nmol min−1 mg protein | 36% (w/w, cell dry weight) of lipids | 46 |
| Ammonium tartrate | 14 nmol min−1 mg protein | 26% (w/w, cell dry weight) | ||
| Mt. alpine (mold) | β-Group vitamins | +63.3% | 1.7 × ARA biosynthesis | 71 |
| Aurantiochytrium sp.SD116 (algae) | Pentanoic acid | <70 U mg−1 | 61.7% to 31.8% DCW of lipid content; 63.4% of TFA of DHA | 72 |
| Isovaleric acid | Low | 61.7% to 37.1% DCW of lipid content; 51.8% of TFA of DHA | ||
| Pentanoic acid be removed | Increased to 220.94 U mg−1 | 0.363 to 0.801 g L−1 h−1 of lipid accumulation; 47.29% of TFA of DHA; 9.5 to 35.8% of TFA of hexadecanoic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
It was hypothesized that down-regulation of the ME gene accounted for the decrease of ME activity in Mc. circinelloides and Mt. alpine. The evidence was that the reinstatement of ME activity by addition of NH4+ was broken by introducing cycloheximide as inhibitor of protein synthesis.23 However, in Cunninghamella sp. 2A1, reduction of ME activity was not due to the down-regulation of ME but the feedback repression after nitrogen starvation. ME activity recovered by adding ammonium tartrate even at a high cyclohexamide concentration, suggesting that the reason for recovery of ME activity was not the afresh synthesized enzyme but the components in ammonium tartrate. Further, there might be some inhibitors in the culture medium to account for the decrease of ME activity.4 Another study inferred that the cease of ME activity at the last stage of lipid accumulation was due to an enzyme that particularly degraded ME in the oleaginous fungi. Researchers presumed that if ME could be transformed to resist degeneration (or if the gene coding the ME-cleaving enzyme could be removed), ME activity should keep completely active so that these two oleaginous fungi could produce more lipid.44
Malic acid could activate ME and was added at the fast lipid production step in oleaginous microalgae Schizochytrium sp.HX-308. Indeed it successfully increased DHA percentage of total lipids but showed little effect on cell growth and overall fatty acids. The enhancement of DHA content could be accounted by the theory that malic acid could make ME change its structure from the dimer to more positive conformations (tetramer or octamer), which provided abundant NADPH for DHA accumulation.20,64 Also, in the R. toruloides CBS 14, the final governance over the ME activity was supposed to be the supply of its substrate. It's a pivotal metabolin that decide how lipid production could be started and maintained.28 Another study of Schizochytrium sp.HX-308 demonstrated that high ME activity could be sustained by phosphate-limitation, leading to enhancement of DHA accumulation. In microalgae, ME might supply NADPH in the later period of leavening when glucose-6-phosphate dehydrogenase (G6PDH) provided NADPH in the early phase (Fig. 5). This might account for the regulatory mechanism of phosphate absence for lipid production and serve more DHA content improvement.65
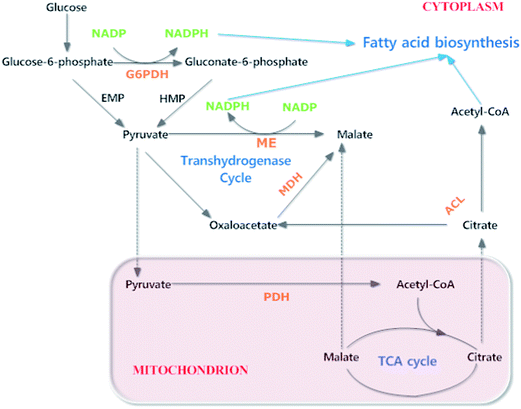 | ||
| Fig. 5 A diagram to show the metabolic pathway of fatty acid biosynthesis and the related key enzymes in microalgae. ACL: ATP: citrate lyase; G6PDH: glucose-6-phosphate dehydrogenase; MDH: malate dehydrogenase; ME: malic enzyme; PDH: pyruvate dehydrogenase.64,65 | ||
The influence of metal ions on ME activity during the lipid production period was obvious. ME from Cunninghamella sp. 2A1 was reported to completely rely on Mg2+ for activity. This accounted for the obvious enhancement in lipid productivity when Mg2+ was added during fermentation, which increased ME activity thus enhancing the NADPH content.66 In Aspergillus niger, adding Cu2+ at the early stage of fermentation to abolish ME activity was an effective strategy to decrease total lipid but increase the citric acid production. It had a more apparent effect when Mg2+ was the unique divalent metal ion in the culture medium. Cu2+ appeared to compete against Mg2+ and Mn2+ for the identical combining site of ME.60
It was reported that using lignocellulosic hydrolysates as carbon sources was a feasible approach for cost-efficient and large-scale lipid production with oleaginous microbes. The introduction of lignocellulosic biomass as a carbon source has advantage of great availability and low cost. In lignocellulosic hydrolysates, four typical alcohol mixtures (furfuryl alcohol, vanillyl alcohol, catechol and hydroquinone) influenced ME activity and lipid production of the yeast Trichosporon fermentans. Except for vanillyl alcohol, all the alcohol compounds inhibited the ME activity, which could account for the depression effect of ethanol compounds on the lipid production of T. fermentans to some extent. Furthermore, the phenomenon that vanillyl alcohol (<25 mM) promoted the lipid production of T. fermentans was due to its little stimulation on ME activity.67 In the previous study, except for furoic acid, other organic acids tested showed inhibitory effect on the ME activity. Furoic acid, similar to vanillyl alcohol, enhanced the lipid production of T. fermentansas probably due to its stimulation on ME activity.68 Also in the former study, among the five typical aldehydes in lignocellulosic hydrolysates tested, syringaldehyde, 4-hydroxybenzaldehyde and furfural had depression effect on ME activity, which could account for the delay of lipid accumulation throughout the zymolysis. Nevertheless, with 5-hydroxymethylfurfural (HMF) and vanillin in the culture medium, the activity of ME didn't change obviously and even an increased ME activity was observed, explaining that the delay of lipid production might result from the reduced specific consumption of glucose and the regulation of cells to the circumstance with restrainers. The change of ME activity was not associated with the delay of lipid production.69
It was reported that ME activity and lipid production with NaNO3 as the nitrogen source was lower than that using organic nitrogen-compounds in Cunninghamella echinulata CCF-103 which produced GLA. As the nitrogen concentration increased, lipid production and ME activity decreased instead. ME might accompany with G6PDH to provide NADPH for fatty acid biosynthesis, but they also channeled more or less NADPH to other metabolic pathways depending upon the kind of nitrogen sources.70 In a study of Mc. circinelloides, the expression of ME isoform IV was motivated by 100% by aspartate, phenylalanine, glutamine and proline at nitrogen exhausted late growth phase. This fungus produced more lipids when using aspartate as nitrogen source instead of ammonium tartrate. It was possibly due to the enhanced ME activity of isoform IV.46
To improve the ARA content in Mt. alpine, researchers found that adding mixed β-group vitamins in the culture medium could increase the specific activity of ME (63.3% higher than the control) and the ARA content could increase to 1.7-fold of the control. It was suggested that β-group vitamins served as the cofactors of the key enzymes or as precursors for the generation of NADPH, and enhanced the concerned metabolic flux. This idea turned out to be a feasible strategy for cost-efficient ARA production and could be utilized in other lipid-producing microbes for more PUFAs.71 Further, researchers discovered that activity of G6PDH was much lower than that of ME, suggesting that the content of NADPH mainly depended on the ME activity. It coincided with the former conclusion that ME particularly supplied the reducing power for lipid accumulation and no other NADPH-generating enzyme could fulfill this role.23,71
It was reported that in Aurantiochytrium sp.SD116 some short-chain fatty acids could influence ME activity and reversibly influence the oil production and fatty acid composition. It was observed that pentanoic acid and isovaleric acid showed great inhibition on the saturated fatty acids biosynthesis from the fatty acid synthase pathway (FAS pathway) by inhibiting the ME activity, while the PUFAs from the polyketide synthase pathway (PKS pathway) was not affected. The enhancement of ME activity after removing the pentanoic acid rapidly reinforced the FAS pathway, which increased the hexadecanoic acid content but decreased DHA production in cells. This demonstrated that the NADPH needed for the PKS pathway might be obtained from other NADPH-producing enzymes, because the productivity of DPA and DHA generated from the PKS pathway didn't decrease along with the weak ME activity. It provided a feasible strategy that suppressing the ME activity or enhancing other NADPH-producing enzyme activity at the metaphase and later stage of accumulation might largely enhance the ultimate DHA content of total fatty acid (TFA).72
Generally, glucose is used as the carbon source for oleaginous microbes to produce lipids. But the cost of glucose accounts for a large proportion in the total medium cost. Low-cost carbon sources should be considered to produce microbial lipids on a large commercial scale.73 In recent studies, volatile fatty acids (VFAs), obtained from agroindustrial lignocellulosic wastes produced from food wastes, were selected as carbon sources in fed-batch cultures.74 A study showed that using VFAs as the carbon source under O2-enriched air supply conditions could obtain high lipid yield.73 We suspect the reason was that the expression of some isoforms of ME, which correlated to lipid accumulation, was induced by high dissolved oxygen tension (DOT) level (a previous research).46 In another study, the yeast could use VFAs as only carbon sources to produce higher lipids in a repeated batch system.75 On the other hand, glycerol is a useful substrate available for oleaginous yeasts to accumulate lipids. A study showed that Rhodotorula glutinis could utilize glycerol alone to produce lipids with very high degree of unsaturated fatty acids. Researcher's interpretation was that glycerol metabolism stimulated the expression of relevant enzymes used in fatty acid synthesis. Microarray analysis would be able to confirm or disprove this theory.76 We suspect that ME's activity was activated by glycerol metabolism probably. On the other hand, xylose as the second most abundant sugar from lignocellulose is an effective carbon source for lipid production too.77 Culture conditions including nitrogen source and concentration, xylose concentration and C/N ratio had effects on the lipid yield.78 Until now, few researches have studied ME's function using VFA, xylose, and glycerol as substrates which are economical materials to produce microbial lipids. With the aim of producing biodiesels more economically and effectively, more trials of regulation of ME using other substrates should be done.
The genetic engineering of ME
The genetic engineering approaches have developed an efflux of metabolites to lipid production by overexpressing some pivotal enzymes in recombinant microalgaes. And the genetic engineering approach is a promising strategy for enhancing lipid production in the long run.79 By studying the relationship between gene expression level and lipid productivity, it was found that ME gene (me g6562) from microalgae Chlorella pyrenoidosa was highly associated with lipid production and might be employed as target gene for genetic modification.80 A study showed an increase in ME expression abundance for oleaginous Chlorella protothecoides cells at the maximum lipid fertility of 820.17 mg per l per day under heterotrophic-iron induction conditions.81 In addition, the consequence of the proteomic analysis of the Chlorella vulgaris emphasized the status for ME as an objective for strain engineering. Also, a combinatorial method that co-expressing ME with other related fatty acid and TAG biosynthetic machinery may supply maximal reducing agent for these energy-consuming courses, or may induce the lipid synthesis in the photodetoxification process.82 In a study of the diatom Phaeodactylum tricornutum cultivated under nitrogen deprivation, the expression of the ME gene, which was located in chloroplast, dramatically increased related to intensive TAG biosynthesis.83 When P. tricornutum ME (PtME) was overexpressed, the expression of PtME as well as its activity was obviously increased. The total lipid content of transgenic P. tricornutum significantly enhanced by 2.5-fold and the neutral lipid content was enhanced by 31% under nitrogen-deficiency condition.84 In spite of few successful cases in lipid overproduction using transgenic microalgae, the studies on lipid pathways and genetic engineering strains for increased lipid accumulation illustrates that ME are one of most hopeful objects for gene transformation.79When the ME gene was identified and cloned, it should then be possible to place it under a constitutive promoter so that an active ME can be synthesized continually after N-limitation. Under such circumstance, lipid accumulation can be increased substantially.85 Cloning and overexpressing ME gene in microorganisms have succeeded in enhancing the cellular lipid in some cases (Table 3). For example, expression of ME in the cytosol in S. cerevisiae has been shown to increase NADPH production.86 In the previous study, the ME from oleaginous fungus Mt. alpina was overexpressed in S. cerevisiae, leading to a little enhancement in ultimate fatty alcohol titer to 98.0 mg L−1. It was the maximal accumulation of fatty alcohols recorded in S. cerevisiae.87 Another study reported that overexpression of ME genes from Mc. circinelloides and Mt. alpine, respectively, in a leucine auxotrophic Mc. circinelloides led to a 2.5-fold enhancement in lipid accumulation. Two to three-fold increase in ME activity were observed in the two transgenic strains. In both cases, the rise of ME activity was associated with a faster lipid accumulation.44 Meantime, the extent of fatty acid desaturation increased a little. Therefore, overexpression of the ME gene promoted both increased of fatty acids and development of PUFAs, e.g. GLA (18:3 n-6). Nevertheless, ME activity was still lost at the later stage of lipid production, implying that some unknown regulators must be there to particularly degrade ME activity (maybe an ME-converting enzyme).44
| Source of ME | Expression host | ME activity | Note | References |
|---|---|---|---|---|
| Mt. alpine | S. cerevisiae | — | Little enhancement in ultimate fatty alcohol titer to 98.0 mg L−1 | 87 |
| Mc. circinelloides and Mt. alpine | Leucine auxotrophic Mc. circinelloides | +2 to 3-fold | +2.5-fold lipid content | 44 |
| Mt. alpine | Mt. alpina | +2-fold | +30% fatty acid content; no change in degree of fatty acid desaturation | 25 |
| Mt. alpine | Mt. alpina | — | +60% ARA content; no change in total fatty acid content | 89 |
| Mc. circinelloides | R. glutinis | +2-fold | +2-fold lipid content | 90 |
| E. coli K12 NADP-ME (maeB) | E. coli BL21 | — | +4-fold lipid content (about 197.74 mg g−1); +5.6-fold lipid content (co-expression of ACC and ME) | 92 |
| E. coli NADP-ME | E. coli BL21 | +826-fold | −15% Fatty acid production | 93 |
| E. coli NAD-ME | E. coli BL21 | — | +2.15-fold fatty acid production | |
| Y. lipolytica | Y. lipolytica | — | Lipid accumulation was not affected | 95 |
| Mt. alpine (mec2) | Y. lipolytica | — | Lipid accumulation was not affected | 51 |
| A modified Y. lipolytica | Y. lipolytica | — | +5–10% lipid content | 96 |
| A. oryzae | A. oryzae | — | Fatty acids production was not affected | 94 |
However, the role of ME in lipid accumulation was challenged by a subsequent study. The above transgenic strains revealed an analogous lipid production of a non-overexpressing prototrophy, implying that another limiting factor of lipid biosynthesis might have arisen after excluding the ME-based bottleneck. It was demonstrated that a fully leucine metabolism pathway rather than ME was essential for fatty acid synthesis in Mc. circinelloides. And the fungi could only utilized the endogenously produced leucine to accumulate lipid, probably concerning the acetyl-CoA synthesis needed for fatty acid biosynthesis.88
But the role of ME was demonstrated in a later study. The gene named malE1 encoding isoform E of ME was cloned and was successfully homologously expressed in Mt. alpina. Overexpression of ME obtained only a 30% enhancement in fatty acid content and a 2-fold increase in ME activity but no change in the extent of fatty acid desaturation. Maybe the enhanced ME activity did not achieve adequate NADPH for fatty acid biosynthesis. And the NADPH formed by ME might be employed by other metabolic reactions.25 In another study of Mt. alpina, homologously overexpressing of the mitochondrial ME (mME) gene achieved 60% increased ARA content without influencing the fatty acid production. The result suggested that increasing mME activity might be an effective strategy to enhance industrial ARA yield in Mt. alpine.89 These two studies demonstrated that ME might not be the unique rate-controlling factor, but it did act a part during lipid accumulation of oil-producing fungi.25
A recent study showed that heterologous expression of ME could facilitate the lipid production of the oleaginous yeast R. glutinis. ME gene from Mc. circinelloides was cloned and successfully overexpressed in oleaginous yeast R. glutinis which obtained a 2-fold enhancement in lipid content. Using the 26S rDNA and 5.8SrDNA gene fragments from R. glutinis could help the ME gene homologously fuse into the chromosome. A strong promoter PGK1 from S. cerevisiae was inserted into the plasmid to obtain steady expression. 2-fold of ME activity was achieved and lipid production increased from 18.74% of the biomass to 39.35%. But no obvious diversity in fatty acid compositions was seen between the wild-type and the recombinant.90 However, the highest lipid content was less than that obtained by other oleaginous microbes. It was probably because the culture medium hadn't been optimized. So the lipid content might be further enhanced after improving the cultivating conditions. In addition, it was inferred that greater enhancement in lipid content could be achieved by co-overexpressing of related lipid-biosynthesis genes.90 In another study of R. toruloides, it was discovered that the transcription of ME was down-regulated while the protein level of ME was apparently enhanced during the lipid production stage, indicating that the regulation of ME activity was sophisticated. The exact mechanism of ME activity in the regulation of lipid biosynthesis are far from clear.91
It was reported that overexpressing NADP-ME gene (maeB) from E. coli K12 in the E. coli BL21 and introducing malate to the culture medium achieved a 4-fold enhancement in lipid content (about 197.74 mg g−1). Further, by co-expression of acetyl-CoA carboxylase (ACC) and NADP-ME, the transformant had a 5.6-fold growth in lipid content relative to the wild-type. This could promote the development of the E. coli to become a fatty acid producer.92 The consequence, however, was unreachable in another study showing that overexpressing the NADP-ME of E. coli BL21 resulted in a 15% decrease in fatty acid production instead. Probably the level of gene overexpression in this study (the ME activity of strain YS3 was 826-fold of that of the control strain YS1) was too high, either burdening the expression host or leading to imbalanced metabolism of the fatty acid biosynthesis. Even so, over-expressing of NAD-ME (EC 1.1.1.38, a NADH producing enzyme) led to a remarkable raise in fatty acid production (2.15-fold of the control), which indicated that NADH could also act as the reductant for lipid accumulation. In addition, NAD-ME possibly acted as a more significant role than any other reducing agent-producing enzymes (NADP-ME, NADP-IDH, G6PD and PGD) in E. coli.93 However, there are some failed examples. In a study of Aspergillus oryzae, researchers constructed a transgenic strain with increased expression of two ME genes (AO090011000876 and AO090038000621) by replacing the original promoters using tef1 promoters of A. oryzae. Nevertheless, contrary to the consequence in Mucor, no enhancement in production of fatty acids was obtained.94
Controversy about the role of ME during lipid biosynthesis
Although ME has been discovered in most oleaginous microorganisms and has been suggested to form a lipogenic metabolon complex with ACL and FAS to facilitate fatty acid elongation, ME–ACL–FAS complex didn't appear to function in all oleaginous yeasts. And NADPH content or ME activity are probably not the rate-limiting steps for lipid production in some yeasts.95In general, lipid biosynthesis of oleaginous microbes happened in the cytosol. Recent studies reported that homologously overexpressing ME (YALI0E18634g) couldn't increase lipid content in wild type Y. lipolytica probably due to ME's low affinity for NADP+ to provide NADPH and its location in the mitochondria. It was implied that ME might not play an important role in lipid production in Y. lipolytica.21,95 In addition, heterologous expression of NADP+-ME from Mt. alpine in Y. lipolytica did not increase the lipid content neither.51 In another study, however, overexpressing the ME gene could enhance the amount of lipid by about 5–10% in a modified Y. lipolytica that the location of ME changed from the mitochondria to the cytosol now. But the problem was that this gene encoded a NAD-ME, indicating that it would not necessarily have produced NADPH for lipid accumulation. But, how it issued in an enhanced lipid content was not clear.96
It was proposed in a recent study that the pentose phosphate pathway (PPP) was the most likely alternative source of NADPH in Y. lipolytica, but some NADPH might come from a cytosolic isocitrate dehydrogenase (ICDH) (NADP+-dependent) reaction. This would be coupled to a mitochondrial ICDH acting in the counterreaction.96 In another study, a 13C-Metabolic Flux Analysis of two Y. lipolytica strains was performed. The metabolic flux distributions were estimated during lipogenesis in a control strain and an engineered strain capable of accumulating lipids at approximately twice the production. The result was that the NADPH-producing flux via the oxidative PPP was roughly doubled in the engineered strain, while the flux via ME did not show obvious difference between the two strains, demonstrating that the lipogenic NADPH was mainly from oxidative PPP for Y. lipolytica to overproduce TAGs from glucose.97 From the above, it seems that ME cannot supply all the reducing power that is required, though it plays a part in most of species.96 The NADPH provided by the MDH-ME cycle might not be essential for lipid accumulation but it might be significant to maintain high lipid productivity.98
Prospects
Taken together, malic enzyme (ME) does play a role in the regulation of fatty acid biosynthesis. Nowadays, energy-saving and environmental protection have been the hot themes worldwide. The biodiesel converted from microbial lipids has often been regarded as an alternative to fossil oil over the past ten years.73 As we have discussed, investigating the regulation of ME on lipid production in oleaginous microorganisms will expect to have good application prospect.Many trials have been done to improve ME's activity during lipid accumulation. It may work by optimizing the culture condition such as adjusting oxygen concentration. Perhaps utilizing other carbon sources available except glucose can improve ME's activity and lipid production as well as decrease the cost of production. Nitrogen source is a key factor that can be optimized too. Also, ME's activity can be improved with various biochemical methods to provide adequate NADPH for fatty acid biosynthesis. On the other hand, those studies on lipid producing approaches and genetic engineering for increased lipid production demonstrate that ME is one of the most hopeful targets for gene transformation.55 Overexpression of ME or co-expression of ME and other enzymes could obviously increase lipid accumulation in transgenic strains. Therefore, more trials of genetic engineering strategy should be done due to its great effectiveness. The exploitation of transgenic strains will open new prospects for the production of biodiesel and may represent a technological breakthrough for biodiesel production.
Although the role of ME during fatty acid synthesis is controversial in some cases, it plays an important part in lipid accumulation of most oleaginous microorganisms. The regulation of ME on lipid accumulation could offer an economic advantage in the production of microbial lipids and biodiesel. And the PUFAs from microbial lipids can be commercially utilized in infant formulae, food, nutritional and pharmaceutical industries. It is believed that in the near future, studies on ME regulating the microbial lipid accumulation will move ahead fast and lipid content will be increased largely.
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ACL | ATP: citrate lyase |
| ARA | Arachidonic acid |
| DHA | Docosahexaenoic acid |
| DOT | Dissolved oxygen tension |
| FAS | Fatty acid synthase |
| GLA | γ-Linolenic acid |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| ICDH | Isocitrate dehydrogenase |
| MaeB | NADP-ME from E. coli |
| MDH | Malate dehydrogenase |
| ME | Malic enzyme |
| m-NAD(P)-ME | Mitochondrial NAD(P)-ME |
| OAA | Oxaloacetic acid |
| PPP | Pentose phosphate pathway |
| PTA | Phosphotransacetylase |
| PUFA | Polyunsaturated fatty acid |
| SCO | Single cell oil |
| SfcA | NAD (P)-ME from E. coli |
| TAG | Triacylglycerol |
| TFA | Total fatty acid |
Acknowledgements
This project was supported by the National Natural Foundation of China (31171631), Guangdong Province Science and technology plan project (2011B031200005), and Guangdong Provincial Bureau of ocean and fishery science and technology to promote a special (A201301C04).References
- X. Meng, J. M. Yang, X. Xu, L. Zhang, Q. J. Nie and M. Xian, Renewable Energy, 2009, 34, 1–5 CrossRef CAS PubMed.
- C. Ratledge, Biochimie, 2004, 86, 807–815 CrossRef CAS PubMed.
- G. F. Chang, Z. L. Luo, S. T. Gu, Q. H. Wu, M. Chang and X. G. Wang, Bioresour. Technol., 2013, 142, 255–260 CrossRef CAS PubMed.
- A. A. Hamid, N. F. Mokhtar, E. M. Taha, O. Omar and W. M. W. Yusoff, Ann. Microbiol., 2011, 61, 463–468 CrossRef PubMed.
- C. Ratledge, S. Hopkins and F. Gunstone, Modif. Lipids Use Food, 2006, 80–113 Search PubMed.
- B. E. Rittmann, Biotechnol. Bioeng., 2008, 100, 203–212 CrossRef CAS PubMed.
- G. Stephanopoulos, Science, 2007, 315, 801–804 CrossRef CAS PubMed.
- Q. Li, W. Du and D. H. Liu, Appl. Microbiol. Biotechnol., 2008, 80, 749–756 CrossRef CAS PubMed.
- X. Y. Qv, Y. Y. Guo and J. G. Jiang, RSC Adv., 2014, 4(79), 42202–42210 RSC.
- X. Y. Qv, Q. F. Zhou and J. G. Jiang, J. Sep. Sci., 2014, 4(79), 2991–2999 CrossRef PubMed.
- C. Ratledge, Biochem. Soc. Trans., 2002, 30, 1047–1049 CrossRef CAS.
- H. Chen, Y. Lu and J. G. Jiang, PLoS One, 2012, 7, e37578 CAS.
- M. H. Liang and J. G. Jiang, Prog. Lipid Res., 2013, 52, 395–408 CrossRef CAS PubMed.
- O. Tehlivets, K. Scheuringer and S. D. Kohlwein, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2007, 1771, 255–270 CrossRef CAS PubMed.
- H. Chen, S. L. Chen and J. G. Jiang, PLoS One, 2011, 6, e28613 CAS.
- G. G. Chang and L. Tong, Biochemistry, 2003, 42, 12721–12733 CrossRef CAS PubMed.
- P. Gourdon, M.-F. Baucher, N. D. Lindley and A. Guyonvarch, Appl. Environ. Microbiol., 2000, 66, 2981–2987 CrossRef CAS.
- Y. H. Li, I. P. Adams, J. P. Wynn and C. Ratledge, Mycol. Res., 2005, 109, 461–468 CrossRef CAS.
- Y. D. Song, J. P. Wynn, Y. H. Li, D. Grantham and C. Ratledge, Microbiology, 2001, 147, 1507–1515 CAS.
- R. T. Wedding, Plant Physiol., 1989, 90, 367–371 CrossRef CAS PubMed.
- A. Beopoulos, J. Cescut, R. Haddouche, J.-L. Uribelarrea, C. Molina-Jouve and J.-M. Nicaud, Prog. Lipid Res., 2009, 48, 375–387 CrossRef CAS PubMed.
- J. P. Wynn and C. Ratledge, Microbiology, 1997, 143, 253–257 CrossRef CAS PubMed.
- J. P. Wynn, A. bin Abdul Hamid and C. Ratledge, Microbiology, 1999, 145, 1911–1917 CrossRef CAS PubMed.
- A. Kendrick and C. Ratledge, Eur. J. Biochem., 1992, 209, 667–673 CrossRef CAS PubMed.
- G. F. Hao, H. Q. Chen, L. Wang, Z. N. Gu, Y. D. Song, H. Zhang, W. Chen and Y. Q. Chen, Appl. Microbiol. Biotechnol., 2014, 80, 2672–2678 Search PubMed.
- J. P. Wynn, A. Kendrick and C. Ratledge, Lipids, 1997, 32, 605–610 CrossRef CAS.
- K. Laoteng, M. Čertík and S. Cheevadhanark, Chem. Pap., 2011, 65, 97–103 CAS.
- C. T. Evans and C. Ratledge, Can. J. Microbiol., 1985, 31, 1000–1005 CrossRef CAS.
- S. Ochoa, A. H. Mehler and A. Kornberg, J. Biol. Chem., 1948, 174, 979 CAS.
- R. M. Zelle, J. C. Harrison, J. T. Pronk and A. J. van Maris, Appl. Microbiol. Biotechnol., 2011, 77, 732–738 CAS.
- W. Fukuda, Y. Sari Ismail, T. Fukui, H. Atomi and T. Imanaka, Archaea, 2005, 1, 293–301 CrossRef CAS PubMed.
- J. Y. Hsieh, S. H. Chen and H. C. Hung, J. Biol. Chem., 2009, 284, 18096–18105 CrossRef CAS PubMed.
- D. E. Coleman, G. J. Rao, E. Goldsmith, P. F. Cook and B. G. Harris, Biochemistry, 2002, 41, 6928–6938 CrossRef CAS PubMed.
- Y. W. Xu, G. Bhargava, H. Wu, G. Loeber and L. Tong, Structure, 1999, 7, 877–889 CrossRef CAS.
- Z. R. Yang, H. L. Zhang, H. C. Hung, C. C. Kuo, L. C. Tsai, H. N. S. Yuan, W. Y. Chou, G. G. Chang and L. Tong, Protein Sci., 2002, 11, 332–341 CrossRef CAS PubMed.
- Z. R. Yang and L. Tong, Protein Pept. Lett., 2000, 7, 287–296 CAS.
- S. Bartolucci, R. Rella, A. Guagliardi, C. Raia, A. Gambacorta, M. De Rosa and M. Rossi, J. Biol. Chem., 1987, 262, 7725–7731 CAS.
- M. C. G. Wheeler, M. A. Tronconi, M. F. Drincovich, C. S. Andreo, U.-I. Flügge and V. G. Maurino, Plant Physiol., 2005, 139, 39–51 CrossRef CAS PubMed.
- I. Sato, J. Yoshikawa, A. Furusawa, K. Chiku, S. Amachi and T. Fujii, Biosci., Biotechnol., Biochem., 2010, 74, 75–81 CrossRef CAS PubMed.
- M. J. Mitsch, R. T. Voegele, A. Cowie, M. Osteras and T. M. Finan, J. Biol. Chem., 1998, 273, 9330–9336 CrossRef CAS PubMed.
- F. P. Bologna, C. S. Andreo and M. F. Drincovich, J. Bacteriol., 2007, 189, 5937–5946 CrossRef CAS PubMed.
- R. K. Wierenga, P. Terpstra and W. G. Hol, J. Mol. Biol., 1986, 187, 101–107 CrossRef CAS.
- T. Vorapreeda, C. Thammarongtham, S. Cheevadhanarak and K. Laoteng, Microbiology, 2013, 159, 2548–2557 CrossRef CAS PubMed.
- Y. Zhang, I. P. Adams and C. Ratledge, Microbiology, 2007, 153, 2013–2025 CrossRef CAS PubMed.
- J. Y. Yang, X. J. Hu, H. Y. Zhang, H. Q. Chen, J. X. Zhao, Y. D. Song, Y. Q. Chen, H. Zhang and W. Chen, Appl. Microbiol. Biotechnol., 2014, 1–9 Search PubMed.
- J. H. Li, H. Y. Zhang and Y. D. Song, Adv. Biomed. Eng., 2011, 3–5 Search PubMed.
- Y. Zhang and C. Ratledge, Mycol. Res., 2008, 112, 725–730 CrossRef CAS PubMed.
- W. Vongsangnak, Y. T. Zhang, W. Chen, C. Ratledge and Y. D. Song, Biotechnol. Lett., 2012, 34, 941–947 CrossRef CAS PubMed.
- A. Abdul Hamid, S. Shuib, E. M Taha, O. Omar, M. S. Khalil, A. J. Abdul Kader and M. H. Mohd Isa, Jurnal Teknologi, 2014, 67(1), 1–5 CrossRef.
- B. Dujon, D. Sherman, G. Fischer, P. Durrens, S. Casaregola, I. Lafontaine, J. De Montigny, C. Marck, C. Neuvéglise and E. Talla, Nature, 2004, 430, 35–44 CrossRef PubMed.
- H. Y. Zhang, L. N. Zhang, H. Q. Chen, Y. Q. Chen, C. Ratledge, Y. D. Song and W. Chen, Biotechnol. Lett., 2013, 35, 2091–2098 CrossRef CAS PubMed.
- W. Tang, S. F. Zhang, H. D. Tan and Z. K. Zhao, Mol. Biotechnol., 2010, 45, 121–128 CrossRef CAS PubMed.
- L. Wang, W. Chen, Y. Feng, Y. Ren, Z. N. Gu, H. Q. Chen, H. C. Wang, M. J. Thomas, B. Zhang and I. M. Berquin, PLoS One, 2011, 6, e28319 CAS.
- Y. M. Zhu, P. P. Zhou, J. R. Hu, R. J. Zhang, L. Ren, M. T. Li, F. Ning, W. Chen and L. J. Yu, PLoS One, 2013, 8, e65552 CAS.
- C. H. Shang, S. N. Zhu, Z. H. Yuan and Z. M. Wang, Adv. Mater. Res., 2012, 347, 2536–2540 Search PubMed.
- M. Saayman, PhD thesis, University of Stellenbosch, 2011.
- J. Savitha, J. Wynn and C. Ratledge, World J. Microbiol. Biotechnol., 1997, 13, 7–9 CrossRef CAS.
- W. Y. Chou, W. P. Tsai, C. C. Lin and G. G. Chang, J. Biol. Chem., 1995, 270, 25935–25941 CrossRef CAS PubMed.
- D. A. Brown and R. A. Cook, Biochemistry, 1981, 20, 2503–2512 CrossRef CAS.
- K. Jernejc and M. Legiša, FEMS Microbiol. Lett., 2002, 217, 185–190 CrossRef CAS PubMed.
- B. T. Driscoll and T. M. Finan, Microbiology, 1997, 143, 489–498 CrossRef PubMed.
- H. C. Hung, G. G. Chang, Z. R. Yang and L. Tong, Biochemistry, 2000, 39, 14095–14102 CrossRef CAS PubMed.
- K. L. Su, K. Y. Chang and H. C. Hung, Bioorg. Med. Chem., 2009, 17, 5414–5419 CrossRef CAS PubMed.
- L. J. Ren, H. Huang, A. H. Xiao, M. Lian, L. J. Jin and X. J. Ji, Bioprocess Biosyst. Eng., 2009, 32, 837–843 CrossRef CAS PubMed.
- L. J. Ren, Y. Feng, J. Li, L. Qu and H. Huang, Bioprocess Biosyst. Eng., 2013, 36, 1177–1183 CrossRef CAS PubMed.
- F. Muhid, W. N. W. Nawi, A. J. Kader, W. M. W. Yusoff and A. A. Hamid, OnLine J. Biol. Sci., 2008, 8, 62 CrossRef CAS.
- C. Huang, H. Wu, L. P. Liu, W. Y. Lou and M. H. Zong, PLoS One, 2012, 7, e46975 CAS.
- C. Huang, H. Wu, Z. J. Liu, J. Cai, W. Y. Lou and M. H. Zong, Biotechnol. Biofuels, 2012, 5(4), 4–10 CrossRef CAS PubMed.
- C. Huang, H. Wu, Q. P. Liu, Y. Y. Li and M. H. Zong, J. Agric. Food Chem., 2011, 59, 4606–4613 CrossRef CAS PubMed.
- M. Certik, J. Megova and R. Horenitzky, J. Gen. Appl. Microbiol., 1999, 45, 289–293 CrossRef CAS.
- Y. Zeng, X. J. Ji, S. M. Chang, Z. K. Nie and H. Huang, Bioprocess Biosyst. Eng., 2012, 35, 683–688 CrossRef CAS PubMed.
- X. J. Song, Y. Z. Tan, Y. J. Liu, J. T. Zhang, G. L. Liu, Y. G. Feng and Q. Cui, J. Agric. Food Chem., 2013, 61, 9876–9881 CrossRef CAS PubMed.
- Q. Fei, H. N. Chang and L. Shang, Biotechnol. Bioprocess Eng., 2011, 16, 482–487 CrossRef CAS.
- P. Fontanille, V. Kumar, G. Christophe, R. Nouaille and C. Larroche, Bioresour. Technol., 2012, 114, 443–449 CrossRef CAS PubMed.
- X. Xu, J. Y. Kim, H. U. Cho, H. R. Park and J. M. Park, Chem. Eng. J., 2015, 264, 735–743 CrossRef CAS PubMed.
- E. R. Easterling, W. T. French, R. Hernandez and M. Licha, Bioresour. Technol., 2009, 100, 356–361 CrossRef CAS PubMed.
- D. Gao, J. Zeng, Y. Zheng, X. Yu and S. Chen, Bioresour. Technol., 2013, 133, 315–321 CrossRef CAS PubMed.
- Q. Fei, S. J. Wewetzer, K. Kurosawa, C. Rha and A. J. Sinskey, Process Biochem., 2015, 18(2), 500–506 CrossRef PubMed.
- N. M. D. Courchesne, A. Parisien, B. Wang and C. Q. Lan, J. Biotechnol., 2009, 141, 31–41 CrossRef CAS PubMed.
- J. H. Fan, Y. B. Cui, M. X. Wan, W. L. Wang and Y. G. Li, Biotechnol. Biofuels, 2014, 7, 17 CrossRef PubMed.
- Y. Li, J. Mu, D. Chen, H. Xu and F. Han, World J. Microbiol. Biotechnol., 2015, 1–11 Search PubMed.
- M. T. Guarnieri, A. Nag, S. H. Yang and P. T. Pienkos, J. Proteomics, 2013, 93, 245–253 CrossRef CAS PubMed.
- Z.-K. Yang, Y.-F. Niu, Y.-H. Ma, J. Xue, M.-H. Zhang, W.-D. Yang, J.-S. Liu, S.-H. Lu, Y. Guan and H.-Y. Li, Biotechnol. Biofuels, 2013, 6, 1–67 CrossRef PubMed.
- J. Xue, Y.-F. Niu, T. Huang, W.-D. Yang, J.-S. Liu and H.-Y. Li, Mebab. Eng., 2015, 27, 1–9 CAS.
- C. Ratledge and J. P. Wynn, Adv. Appl. Microbiol., 2002, 51, 1–51 CAS.
- M. Moreira dos Santos, V. Raghevendran, P. Kötter, L. Olsson and J. Nielsen, Metab. Eng., 2004, 6, 352–363 CrossRef CAS PubMed.
- W. Runguphan and J. D. Keasling, Mebab. Eng., 2014, 21, 103–113 CrossRef CAS PubMed.
- R. A. Rodríguez-Frómeta, A. Gutiérrez, S. Torres-Martínez and V. Garre, Appl. Microbiol. Biotechnol., 2013, 97, 3063–3072 CrossRef PubMed.
- G. F. Hao, H. Q. Chen, K. Du, X. Y. Huang, Y. D. Song, Z. N. Gu, L. Wang, H. Zhang, W. Chen and Y. Q. Chen, Biotechnol. Lett., 2014, 1–8 CAS.
- Z. Li, H. X. Sun, X. M. Mo, X. Y. Li, B. Xu and P. Tian, Appl. Microbiol. Biotechnol., 2013, 97, 4927–4936 CrossRef CAS PubMed.
- Z. W. Zhu, S. F. Zhang, H. W. Liu, H. W. Shen, X. P. Lin, F. Yang, Y. J. Zhou, G. J. Jin, M. L. Ye and H. F. Zou, Nat. Commun., 2012, 3, 1112 CrossRef PubMed.
- X. Meng, J. M. Yang, Y. J. Cao, L. Z. Li, X. L. Jiang, X. Xu, W. Liu, M. Xian and Y. W. Zhang, J. Ind. Microbiol. Biotechnol., 2011, 38, 919–925 CrossRef CAS PubMed.
- Y. S. Zhou, H. Y. Zhang, H. Q. Chen, Y. D. Song, Y. Q. Chen, H. Zhang and W. Chen, Sciencepaper online http://www.paper.edu.cn/html/releasepaper/2013/03/716/, 2013.
- K. Tamano, K. S. Bruno, S. A. Karagiosis, D. E. Culley, S. Deng, J. R. Collett, M. Umemura, H. Koike, S. E. Baker and M. Machida, Appl. Microbiol. Biotechnol., 2013, 97, 269–281 CrossRef CAS PubMed.
- A. Beopoulos, J.-M. Nicaud and C. Gaillardin, Appl. Microbiol. Biotechnol., 2011, 90, 1193–1206 CrossRef CAS PubMed.
- C. Ratledge, Biotechnol. Lett., 2014, 1–12 Search PubMed.
- T. M. Wasylenko, W. S. Ahn and G. Stephanopoulos, Mebab. Eng., 2015, 30(1), 27–39 Search PubMed.
- A. Ochoa-Estopier and S. E. Guillouet, J. Biotechnol., 2014, 170, 35–41 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |


