The role of H3PO4 in the preparation of activated carbon from NaOH-treated rice husk residue
Yaxin Li,
Xian Zhang,
Ruiguang Yang,
Guiying Li and
Changwei Hu*
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, Sichuan 610064, China. E-mail: gchem@scu.edu.cn; chwehu@mail.sc.cninfo.net; changweihu@scu.edu.cn; Fax: +86-28-85411105; Tel: +86-28-85411105
First published on 31st March 2015
Abstract
The preparation of activated carbon from rice husk residue using H3PO4 as activation agent was studied. The samples were characterized by elemental analysis, N2 adsorption–desorption, scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), temperature programmed decomposition-mass spectra (TPD-MS), thermogravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS). The role of H3PO4 in the activation process was discussed. A maximum surface area of 1016 m2 g−1 was obtained under the optimized conditions, that is, a base treated solid material to H3PO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, an activation temperature of 500 °C and an activation time of 1 h. H3PO4 might act as a catalyst which facilitates the release of CO2, an oxidant which reacts with carbon after dehydration, and a reagent which enters AC through C–O–P bonds.
2, an activation temperature of 500 °C and an activation time of 1 h. H3PO4 might act as a catalyst which facilitates the release of CO2, an oxidant which reacts with carbon after dehydration, and a reagent which enters AC through C–O–P bonds.
1. Introduction
Rice is regarded as one of the most important agricultural crops in Asia and the annual quantity of rice husk is very large as a by-product from rice mills. China is the most important rice producer in the world, with an annual output of approximately 200 million tons, whereas most of the rice husk is discarded or directly burned up traditionally.1 Some researchers have paid attention to the conversion of rice husk into bio-oil,2,3 and large amount of solid residue named rice husk residue (RHR) is generated at the same time. The utilization of the abundant RHR to produce useful materials is of great significance, not only to avoid the environmental pollution produced from combustion, but also to provide plenty economic benefits.Activated carbon (AC) is a carbonaceous material with highly developed porosity, and as a result it is generally used as adsorbent, catalyst, and catalyst support.4 Rice husk from rice mill or rice husk ash after burning from rice husk in paper mill had been used for the production of AC. However, RHR is an interesting potential starting material owing to its low cost. There are only few references reporting its use in AC production.4–6 The surface area of AC prepared from RHR is usually low because of the high ash content,7 whereas the ash can be removed by base treatment.
Physical and chemical activation of the starting materials are the two fundamentally employed methods for the preparation of AC. Physical activation involves the carbonization of a precursor using a gaseous activating agent.8 Chemical activation mixes the precursor with a chemical activating agent, and then heats it in an inert gas. A comparison of chemical activation with physical activation shows that chemical activation needs a lower reaction temperature.7 Phosphoric acid, zinc chloride and potassium hydroxide are used extensively as activating agents, and there are some differences between them. KOH produces a widening of micropore width, while ZnCl2 develops small mesoporosity, and H3PO4 leads to a more heterogeneous pore size distribution.9 H3PO4 has become a common chemical activating agent used for preparing AC from a variety of starting materials,10–12 because of the lower environmental and toxicological constrains compared to ZnCl2, and lower working temperature compared with KOH or NaOH.12 As reported before, the best activation temperature was 800 °C for rice husk activated by NaOH13 and 900 °C for olive husk activated by KOH,14 and there was a carbonization step before activation process. Additionally, the corrosiveness of the process should also be taken into consideration, especially with strong base as the activator, which exhibits serious corrosion activity toward the reactor. Additionally, the recycled acid could also be used for neutralizing the solution in the base treatment process, producing silica. It is well known that H3PO4 appears to function as both an acid catalyst to promote bond cleavage reactions and a reactant in the formation of crosslinks via process, such as cyclization and condensation. H3PO4 could also combine with organic species in biomass waste to form phosphate and polyphosphate bridges that connect and crosslink polymer fragments.15 Furthermore, some of these phosphate groups remain on the carbon surface after the washing step.16 According to Liou et al.,7 the activation process of rice husk is divided into three stage: the organic matter decomposes into the intermediate of smaller molar mass and releases gaseous volatiles in the initial stage of the activation reaction; the intermediate further decomposes to form other volatile species, tar and char, and H3PO4 begin to decompose at the same time; in the third stage, the char reacts with P2O5, causing the pores to open. However, the study of the function of H3PO4 is not as clear as possible.
The aim of the present work is to take advantage of by-product RHR to produce AC and study the function of H3PO4 in the activation process.
2. Experimental
2.1. Materials
RHR was produced from rice husk in a fluidized bed reactor, where rice husk was exposed to 475 °C for less than 2 s to get bio-oil for the purpose of energy material.17 Sodium hydroxide (NaOH) and phosphoric acid (H3PO4, 85%) were of analytical grade and applied without further purification. N2 was of chemical grade (at a purity of 99.9%). Deionized water was employed throughout all the treatment processes.2.2. Preparation of activated carbon
RHR (40–80 mesh) was washed with deionized water to remove the impurities, and then dried in an oven at 100 °C for 24 h. 30 g of dried rice husk residue was put into a flask, and 200 mL of 3 mol L−1 NaOH solution18 was added into the reactor, then the temperature was increased to 105 °C and kept for 5 h. NaOH reacted with silica to form soluble Na2SiO3. The suspension was filtrated and the base treated solid residue (abbreviated as BTRHR) was washed with deionized water, and then dried for use in the preparation of AC.2 g of BTRHR was impregnated with 15 mL H3PO4 aqueous solution at various BTRHR/H3PO4 ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 1
2.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The impregnated residue was set into an oven at 100 °C for one night to remove the excess water, and then the dried sample (PBTRHR) was used for activation where it was heated from room temperature at 5 °C min−1 up to various activation temperature for different activation time under N2 flow of 60 mL min−1 in a horizontal cylindrical furnace. The resultant sample was washed with deionized water until neutral and dried to obtain AC. The washing water was collected for neutralizing the filtrate of the first step, producing silica. The obtained AC samples were named according to the preparation condition, for example, AC (1
3). The impregnated residue was set into an oven at 100 °C for one night to remove the excess water, and then the dried sample (PBTRHR) was used for activation where it was heated from room temperature at 5 °C min−1 up to various activation temperature for different activation time under N2 flow of 60 mL min−1 in a horizontal cylindrical furnace. The resultant sample was washed with deionized water until neutral and dried to obtain AC. The washing water was collected for neutralizing the filtrate of the first step, producing silica. The obtained AC samples were named according to the preparation condition, for example, AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) meant that the sample was obtained with BTRHR/H3PO4 mass ratio of 1
2/1/500) meant that the sample was obtained with BTRHR/H3PO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, an activation time of 1 h and an activation temperature of 500 °C.
2, an activation time of 1 h and an activation temperature of 500 °C.
2.3. Characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500). They were recorded in the region of 4000–400 cm−1 employing the KBr pellet method.
2/1/500). They were recorded in the region of 4000–400 cm−1 employing the KBr pellet method.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was put in a stainless steel Utube. Pure He sweeping was performed at room temperature until the baseline of MS signals stabilized. TPD-MS was conducted from 30 to 800 °C at a ramping of 5 °C min−1 under He flow rate of 30 mL min−1. The possible molecules (CO2, PH3, O2, CO and H2O) released were monitored with MS signals of m/z = 44, 34, 28, and 18. BTRHR was taken out as a reference.
2) was put in a stainless steel Utube. Pure He sweeping was performed at room temperature until the baseline of MS signals stabilized. TPD-MS was conducted from 30 to 800 °C at a ramping of 5 °C min−1 under He flow rate of 30 mL min−1. The possible molecules (CO2, PH3, O2, CO and H2O) released were monitored with MS signals of m/z = 44, 34, 28, and 18. BTRHR was taken out as a reference.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) were also heated to 800 °C at a ramp of 5 °C min−1 in air to get an insight of the change after base treatment and acid activation.
2/1/500) were also heated to 800 °C at a ramp of 5 °C min−1 in air to get an insight of the change after base treatment and acid activation.3. Results and discussion
3.1. Characterization of the samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) were shown in Table 1. The relative percentage of carbon, hydrogen and nitrogen elements increased after base treatment because of the removal of silica. Then, the percentage of carbon element was increased after activation process. However, hydrogen and nitrogen elements followed the opposite variation trend. The decrease in hydrogen and nitrogen may be attributed to the formation of vapor, ammonia or other substance. Similar results were reported by Liou et al.7 The rest components of the samples were oxygen and ash.
2/1/500) were shown in Table 1. The relative percentage of carbon, hydrogen and nitrogen elements increased after base treatment because of the removal of silica. Then, the percentage of carbon element was increased after activation process. However, hydrogen and nitrogen elements followed the opposite variation trend. The decrease in hydrogen and nitrogen may be attributed to the formation of vapor, ammonia or other substance. Similar results were reported by Liou et al.7 The rest components of the samples were oxygen and ash.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500)
2/1/500)
| Sample name | RHR | BTRHR | AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) 2/1/500) |
|---|---|---|---|
| Elemental composition (wt%) | |||
| C | 42.00 | 67.20 | 70.62 |
| H | 2.08 | 3.09 | 2.09 |
| N | 0.34 | 0.65 | 0.56 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| N2 adsorption–desorption | |||
| SBET (m2 g−1) | 96 | 201 | 1016 |
| Vt (cm3 g−1) | 0.059 | 0.13 | 0.53 |
| Vmic (cm3 g−1) | 0.031 | 0.055 | 0.31 |
| Vmes (cm3 g−1) | 0.028 | 0.075 | 0.22 |
According to N2 adsorption–desorption results, the surface area, the total pore volume, microporous volume and the mesoporous volume increased dramatically after base treatment and activation process. It could be concluded that a porous structure was leaving behind after the removal of silica. Moreover, H3PO4 activation process made more porous structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
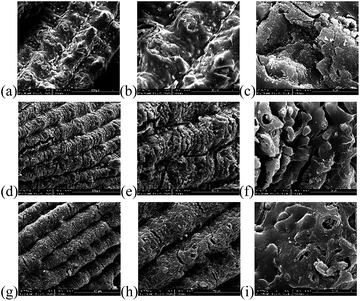
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) with different magnifications. From Fig. 1a–c the corrugated epidermis could be observed, and there is no noticeable difference between RHR and rice husk. New pores formed with the release of silica by the base treatment according to Fig. 1d–f. The pictures of AC (1
2/1/500) with different magnifications. From Fig. 1a–c the corrugated epidermis could be observed, and there is no noticeable difference between RHR and rice husk. New pores formed with the release of silica by the base treatment according to Fig. 1d–f. The pictures of AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) was shown in Fig. 1g–i.
2/1/500) was shown in Fig. 1g–i.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) were shown in Fig. 2. Fig. 2a showed a characteristic peak of silica, which corresponded to the presence of cristobalite in ash.1 After base treatment and H3PO4 activation, BTRHR and AC (1
2/1/500) were shown in Fig. 2. Fig. 2a showed a characteristic peak of silica, which corresponded to the presence of cristobalite in ash.1 After base treatment and H3PO4 activation, BTRHR and AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) showed two vital peaks at 25 and 42°, indicating the existence of the (002) and (100) planes, of the graphitic structure, respectively.19,20
2/1/500) showed two vital peaks at 25 and 42°, indicating the existence of the (002) and (100) planes, of the graphitic structure, respectively.19,20
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) vibration and the band around 1400 cm−1 could be assigned to ν(C–O) vibration in the carboxylate group.23 From 1400 to 400 cm−1, there were some differences between the samples. RHR had three bands around 1090, 798 and 460 cm−1. The results were probably caused by the silicon atom initially attached to the oxygen atom.24 However, BTRHR didn't have these bands, indicating the removal of silica.
C) vibration and the band around 1400 cm−1 could be assigned to ν(C–O) vibration in the carboxylate group.23 From 1400 to 400 cm−1, there were some differences between the samples. RHR had three bands around 1090, 798 and 460 cm−1. The results were probably caused by the silicon atom initially attached to the oxygen atom.24 However, BTRHR didn't have these bands, indicating the removal of silica.
The adsorption around 1180 cm−1 of AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) might be assigned to the stretching mode of hydrogen-bonded P
2/1/500) might be assigned to the stretching mode of hydrogen-bonded P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, to O–C stretching vibrations in P–O–C (aromatic) linkage and to P
O, to O–C stretching vibrations in P–O–C (aromatic) linkage and to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OOH.25
OOH.25
3.2. Optimization of preparation conditions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with activation time of 1 h. As shown in Table 2, the activation temperature exhibited significant influence on the textural properties. The surface area and pore volume gradually increased with activation temperature from 300 to 500 °C. At 500 °C, the sample showed remarkably improved porosity compared with the samples activated at other activation temperature, with a maximum surface area and total pore volume of 1016 m2 g−1 and 0.53 cm3 g−1, respectively. However, the surface area and pore volume decreased with further increase in temperature from 500 to 700 °C.
2 with activation time of 1 h. As shown in Table 2, the activation temperature exhibited significant influence on the textural properties. The surface area and pore volume gradually increased with activation temperature from 300 to 500 °C. At 500 °C, the sample showed remarkably improved porosity compared with the samples activated at other activation temperature, with a maximum surface area and total pore volume of 1016 m2 g−1 and 0.53 cm3 g−1, respectively. However, the surface area and pore volume decreased with further increase in temperature from 500 to 700 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the activation time of 1 h
2 and the activation time of 1 h
| Activation temperature (°C) | SBET (m2 g−1) | Vt (cm3 g−1) | Vmic (cm3 g−1) | Vmes (cm3 g−1) |
|---|---|---|---|---|
| 300 | 633 | 0.33 | 0.19 | 0.14 |
| 400 | 910 | 0.47 | 0.28 | 0.19 |
| 500 | 1016 | 0.53 | 0.31 | 0.22 |
| 600 | 996 | 0.50 | 0.31 | 0.19 |
| 700 | 651 | 0.32 | 0.21 | 0.11 |
At low activation temperature of 300 and 400 °C, the pore structure might not fully develop.7 However, when the temperature was higher than 500 °C, violent gasification reactions might cause part of the micropore structure to be destroyed by collapsing or combining together.26
Fig. 4a showed the N2 adsorption–desorption isotherms of AC prepared at different temperature. According to the IUPAC classification,27 these isotherms seemed to be Type I, which means AC was microporous. When the relative pressure increased above 0.4, the isotherms showed hysteresis loops which were the Type H4 loop. The DFT pore size distributions of AC prepared at different temperature were shown in Fig. 4b. The sample produced at activation temperature of 500 °C showed the strongest pore width at 10 Å. It could be concluded that 500 °C was the optimum activation temperature. The best activation temperature of 500 °C was reported for AC preparation from different waste such as grape seeds,12 rice husk26 and bituminous coal.28 Other optimized activation temperature for AC production from rice hull21 and rice husk19 at 400 and 900 °C were also reported. The material of this study was the residue of rice husk which had been used in bio-oil production. Although it was different from these original wastes, the best activation temperature was still 500 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the surface area of AC increased from 553 m2 g−1 at a ratio of 1
3, the surface area of AC increased from 553 m2 g−1 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reached a maximum of 1016 m2 g−1 at a ratio of 1
1 and reached a maximum of 1016 m2 g−1 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and then decreased. The total pore volume, microporous volume and the mesoporous volume followed the same tendency as the surface area.
2, and then decreased. The total pore volume, microporous volume and the mesoporous volume followed the same tendency as the surface area.
| BTRHR/H3PO4 ratio | SBET (m2 g−1) | Vt (cm3 g−1) | Vmic (cm3 g−1) | Vmes (cm3 g−1) |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
553 | 0.29 | 0.17 | 0.12 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
886 | 0.46 | 0.27 | 0.19 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1016 | 0.53 | 0.31 | 0.22 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
773 | 0.40 | 0.23 | 0.17 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
543 | 0.29 | 0.16 | 0.13 |
The inadequate development of porous structure was observed at the BTRHR/H3PO4 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. This might be due to the insufficient amount of phosphoric acid, which could not activate the sample effectively. However, when the BTRHR/H3PO4 ratio was higher than 1
1.5. This might be due to the insufficient amount of phosphoric acid, which could not activate the sample effectively. However, when the BTRHR/H3PO4 ratio was higher than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the surface area and pore volume decreased. This observation was probably due to the phosphate and polyphosphate species which are incorporated into the carbon matrix through C–O–P bonds.30 Part of the porosity of AC was blocked by phosphorus compounds, which are not easily removed with washing.7
2, the surface area and pore volume decreased. This observation was probably due to the phosphate and polyphosphate species which are incorporated into the carbon matrix through C–O–P bonds.30 Part of the porosity of AC was blocked by phosphorus compounds, which are not easily removed with washing.7
N2 adsorption–desorption isotherms and pore size distributions of the samples activated at different BTRHR/H3PO4 ratios were shown in Fig. 4c and d. All the samples displayed Type I isotherms and H4 hysteresis loop. The sample produced at a BTRHR/H3PO4 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 showed the strongest pore width at 10 Å compared with other BTRHR/H3PO4 ratio. It could be concluded that an appropriate BTRHR/H3PO4 ratio was beneficial to the formation of the pore structure. Consequently, keeping the BTRHR/H3PO4 ratio at 1
2 showed the strongest pore width at 10 Å compared with other BTRHR/H3PO4 ratio. It could be concluded that an appropriate BTRHR/H3PO4 ratio was beneficial to the formation of the pore structure. Consequently, keeping the BTRHR/H3PO4 ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 could lead to a favorable development of the porosity in AC. Other rice husk to phosphoric acid ratios such as 1
2 could lead to a favorable development of the porosity in AC. Other rice husk to phosphoric acid ratios such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (85% of phosphoric acid),7 1
2 (85% of phosphoric acid),7 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (50% of phosphoric acid),31 and 1
5 (50% of phosphoric acid),31 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2 (100% of phosphoric acid)19 were reported. Additionally, jackfruit peel waste to phosphoric acid ratio and waste tires to phosphoric acid ratio of 1
4.2 (100% of phosphoric acid)19 were reported. Additionally, jackfruit peel waste to phosphoric acid ratio and waste tires to phosphoric acid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (85% of phosphoric acid)32 and 1
4 (85% of phosphoric acid)32 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (100% of phosphoric acid)33 were reported, respectively. The BTRHR/H3PO4 mass ratio of 1
5 (100% of phosphoric acid)33 were reported, respectively. The BTRHR/H3PO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (85% of phosphoric acid) in our study was lower than or equal to the value in reported studies.
2 (85% of phosphoric acid) in our study was lower than or equal to the value in reported studies.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. As shown in Table 4, the textural properties increased from 1007 m2 g−1 for 0.75 h and reached a maximum of 1016 m2 g−1 for 1 h, and then decreased. When activated for 0.75 h, the pore structure was inadequately developed and had inferior textural properties, denoting incomplete reaction between BTRHR and H3PO4. When activated for 1 h, the sample exhibited the best pore structure development, indicating that this activation time enabled sufficient reaction. However, when activated for 1.5 and 2 h, the surface area and porosity decreased because the excessive reaction damaged the pores that had already formed. Fig. 4e and f displayed the adsorption–desorption isotherms and DFT pore size distributions of AC. It could be concluded that the activation time of 1 h can be considered as a suitable point. Similar behavior (activated for 1 h) was observed for the materials of rice husk and corncob.10,26,34 Other materials such as rice straw,11 hemp stem and olive stones16 needed 2 h activation to prepare AC.
2. As shown in Table 4, the textural properties increased from 1007 m2 g−1 for 0.75 h and reached a maximum of 1016 m2 g−1 for 1 h, and then decreased. When activated for 0.75 h, the pore structure was inadequately developed and had inferior textural properties, denoting incomplete reaction between BTRHR and H3PO4. When activated for 1 h, the sample exhibited the best pore structure development, indicating that this activation time enabled sufficient reaction. However, when activated for 1.5 and 2 h, the surface area and porosity decreased because the excessive reaction damaged the pores that had already formed. Fig. 4e and f displayed the adsorption–desorption isotherms and DFT pore size distributions of AC. It could be concluded that the activation time of 1 h can be considered as a suitable point. Similar behavior (activated for 1 h) was observed for the materials of rice husk and corncob.10,26,34 Other materials such as rice straw,11 hemp stem and olive stones16 needed 2 h activation to prepare AC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2
| Activation time (h) | SBET (m2 g−1) | Vt (cm3 g−1) | Vmic (cm3 g−1) | Vmes (cm3 g−1) |
|---|---|---|---|---|
| 0.75 | 1007 | 0.51 | 0.31 | 0.20 |
| 1 | 1016 | 0.53 | 0.31 | 0.22 |
| 1.25 | 987 | 0.51 | 0.30 | 0.21 |
| 1.5 | 947 | 0.49 | 0.29 | 0.20 |
| 2 | 865 | 0.45 | 0.26 | 0.19 |
Overall, an activation temperature of 500 °C, a BTRHR/H3PO4 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and an activation time of 1 h were selected as the most adaptable condition for the preparation of AC.
2 and an activation time of 1 h were selected as the most adaptable condition for the preparation of AC.
3.3. The role of H3PO4 in the activation process
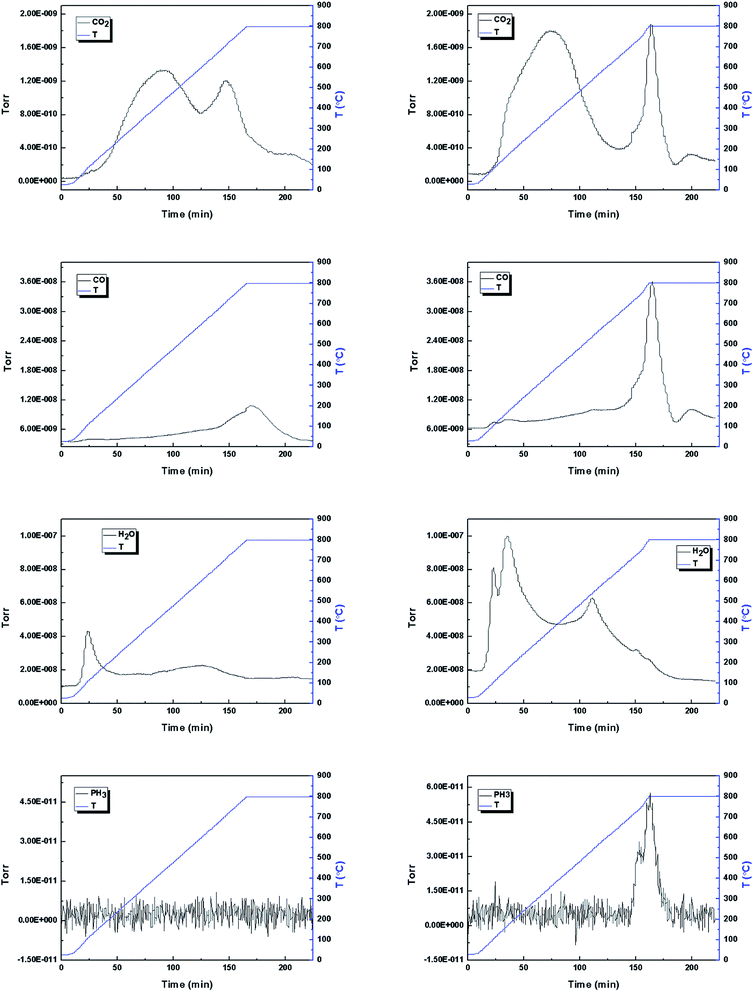
A gently increase of CO could be seen from the spectra of BTRHR from 110 to 800 °C and then decreased in the constant stage. This could be assigned to the decomposition of carbonyl, quinone and/or ether oxygenated species.35 A gently increase of CO desorption of PBTRHR took place from 110 to 700 °C and then a sharp increase of CO could be seen from the spectra of PBTRHR above 700 °C. During the constant stage the spectra decreased as well. According to the CO spectra of the samples, some reactions might exist above 700 °C for PBTRHR.
Fig. 5 showed the evolution of H2O. BTRHR exhibited a peak at about 105 °C, ascribed to desorption of adsorbed water. At higher temperature, BTRHR displayed a low and continuous release of H2O with a gentle peak. PBTRHR showed three peaks developed with maxima at 105 and 170 °C below 400 °C, and another peak with maxima at 530 °C between 400 and 800 °C. These peaks should be due to the dehydration of H3PO4.
PH3 spectra of BTRHR showed no PH3 produced in the process, while, the formation of PH3 could be seen at higher temperature (>700 °C) for PBTRHR. The peak increased from 700 to 800 °C and then decreased during the constant stage, indicating reductive reaction occurred. The process made the valence state of phosphorus reduced from +5 to −3.
The TGA results of BTRHR and PBTRHR were illustrated in Fig. 6. From 100 to 400 °C, the mass loss of 7.7% for BTRHR and the mass loss of 16.4% for PBTRHR mainly corresponded to the release of H2O, CO2 and a small amount of CO. In the temperature range of 400–700 °C and 700–800 °C, the mass loss of 14.8% and 3.9% of BTRHR was attributed to the release of H2O, CO2 and CO. The mass loss of PBTRHR at 400–700 °C corresponded to the activation process, in which the mass loss of 30.4% might be attributed to the reaction between the activating agent and carbonaceous residue,7 producing H2O, CO2 and CO. From 700 to 800 °C, the mass loss of 7.6% should be due to the release of CO2, CO and PH3.
Fig. 7 showed the TGA (a) and DTG (b) curves of RHR, BTRHR and AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) in air flow. The mass loss of RHR and BTRHR occurred in the range from 250 to 650 °C, whereas the mass loss of AC occurred in the range from 400 to 800 °C. This indicated that the base treatment did not change the oxidation performance of the samples obviously according to Fig. 7b. However, H3PO4 activation process changed the oxidation performance. The peak oxidation temperature increased from 423 °C for BTRHR to 560 °C for AC (1
2/1/500) in air flow. The mass loss of RHR and BTRHR occurred in the range from 250 to 650 °C, whereas the mass loss of AC occurred in the range from 400 to 800 °C. This indicated that the base treatment did not change the oxidation performance of the samples obviously according to Fig. 7b. However, H3PO4 activation process changed the oxidation performance. The peak oxidation temperature increased from 423 °C for BTRHR to 560 °C for AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500). It could be due to the fact that the more highly cross-linked structure was developed after activated by H3PO4, which was less prone to volatile loss.28
2/1/500). It could be due to the fact that the more highly cross-linked structure was developed after activated by H3PO4, which was less prone to volatile loss.28
Besides, the ash content decreased after the base treatment compared with RHR because of the ash removal of silica. However, the ash content was increased after activated by H3PO4 compared to BTRHR. The reason of the high ash content of AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/1/500) might be linked to the mechanism of H3PO4 activation. Phosphoric acid might combine with organic species to form phosphoric linkages, such as phosphate and polyphosphate esters, that result in broader porous structures in AC.15 Moreover, the incorporated phase contained not only phosphoric acid but a mixture of polyphosphoric acids, including predominant species such as H3PO4, H4P2O7 and H5P3O10 and some others in lower proportion (e.g. Hn+2PnO3n+1) after activation process.28 The acids could be collected through washing for further use. However, some of these phosphate groups remain on the carbon surface after the washing step, as reported in previous study.16 Consequently, the ash content was increased after being activated by H3PO4.
2/1/500) might be linked to the mechanism of H3PO4 activation. Phosphoric acid might combine with organic species to form phosphoric linkages, such as phosphate and polyphosphate esters, that result in broader porous structures in AC.15 Moreover, the incorporated phase contained not only phosphoric acid but a mixture of polyphosphoric acids, including predominant species such as H3PO4, H4P2O7 and H5P3O10 and some others in lower proportion (e.g. Hn+2PnO3n+1) after activation process.28 The acids could be collected through washing for further use. However, some of these phosphate groups remain on the carbon surface after the washing step, as reported in previous study.16 Consequently, the ash content was increased after being activated by H3PO4.
 | ||
| Fig. 8 XPS spectra of P 2p peak of (a) PBTRHR and the samples (b–f) activated at 300, 400, 500, 600 and 700 °C. | ||
The O 1s spectra of PBTRHR and the samples activated by H3PO4 at 300, 400, 500, 600, 700 °C was shown in Fig. 9. A satisfactory fit was achieved by means of four components: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and non-bridging oxygen in the phosphate group (P
O groups and non-bridging oxygen in the phosphate group (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bonds (peak 1, 531.5 eV); singly bonded oxygen (–O–) in C–OH, C–O–C and/or C–O–P (peak 2, 531.9–532.3 eV); P–O–P bonds (peak 3, 533.2 eV); chemisorbed oxygen and water (peak 4, 532.7–533.9 eV).37–39 According to Table 5, the relative content of peak 1 was reduced with the increase of activation temperature, especially from 500 to 700 °C. The reduction of the relative content of peak 1 might have correlation with the release of CO2 and/or CO with the increase of temperature and the reductive reaction between phosphorus compounds and BTRHR. The relative content of peak 3 was increased as the temperature rose. The dehydration of phosphoric acid and the change of the structure of phosphorus complexes should be the reason of the variation of relative content of peak 3. The relative content of peak 2 decreased firstly and reached a minimum of 15.4%, and increased subsequently. Three reasons might affect the variation of the relative content of peak 2: first, the phosphorous compound linked with BTRHR through C–O–P bond; second, the release of CO; third, the phosphorus complexes react with other adjacent groups, forming P4O10 and polyphosphates groups.16,40 Furthermore, the extra water washing after activation might cause reaction between phosphorus residue and water, and thus influence the content of peak 2 and 3. For example, P4O6 will react with H2O to form phosphorous acid again and dissolved during washing.
O) bonds (peak 1, 531.5 eV); singly bonded oxygen (–O–) in C–OH, C–O–C and/or C–O–P (peak 2, 531.9–532.3 eV); P–O–P bonds (peak 3, 533.2 eV); chemisorbed oxygen and water (peak 4, 532.7–533.9 eV).37–39 According to Table 5, the relative content of peak 1 was reduced with the increase of activation temperature, especially from 500 to 700 °C. The reduction of the relative content of peak 1 might have correlation with the release of CO2 and/or CO with the increase of temperature and the reductive reaction between phosphorus compounds and BTRHR. The relative content of peak 3 was increased as the temperature rose. The dehydration of phosphoric acid and the change of the structure of phosphorus complexes should be the reason of the variation of relative content of peak 3. The relative content of peak 2 decreased firstly and reached a minimum of 15.4%, and increased subsequently. Three reasons might affect the variation of the relative content of peak 2: first, the phosphorous compound linked with BTRHR through C–O–P bond; second, the release of CO; third, the phosphorus complexes react with other adjacent groups, forming P4O10 and polyphosphates groups.16,40 Furthermore, the extra water washing after activation might cause reaction between phosphorus residue and water, and thus influence the content of peak 2 and 3. For example, P4O6 will react with H2O to form phosphorous acid again and dissolved during washing.
 | ||
| Fig. 9 XPS spectra of O1s peak of (a) PBTRHR and the samples (b–f) activated at 300, 400, 500, 600 and 700 °C. | ||
| Samples | Peak 1 | Peak 2 | Peak 3 | Peak 4 |
|---|---|---|---|---|
| (a) | 15.9 | 34.5 | 9.8 | 39.8 |
| (b) | 15.5 | 19.8 | 17.7 | 47.0 |
| (c) | 15.4 | 15.4 | 29.4 | 39.8 |
| (d) | 14.3 | 21.7 | 32.9 | 31.1 |
| (e) | 11.7 | 23.4 | 33.5 | 31.4 |
| (f) | 9.2 | 23.8 | 35.1 | 31.9 |
3.4. Discussion
According to TPD-MS, TGA-DTG, XPS and the structure of phosphorous compound, main reactions which might take place over different temperature range can be supposed as the following.From 100 to 400 °C, the following reactions might occur:
| 2H3PO4 → H4P2O7 + H2O | (1) |
| 3H3PO4 → H5P3O10 + 2H2O | (2) |
| nH3PO4 → Hn+2PnO3n+1 + (n − 1)H2O | (3) |
The structure of phosphorous compound varied with the increase of temperature, as shown in the H2O spectra of TPD-MS and the O 1s of XPS. Two peaks and the continuously release of H2O were shown in the H2O spectra below 400 °C. It was the dehydration of H3PO4, as shown in the reaction (1)–(3). As a result, the content of peak 3 (P–O–P) was increased rapidly from (a) PBTRHR to (c) the sample activated at 400 °C according to Table 5. Furthermore, H3PO4 might act as a catalyst, making the release of CO2 at lower temperature. The content of peak 1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P
O, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) decreased slightly, which might be due to the production of CO2 decomposed from C
O) decreased slightly, which might be due to the production of CO2 decomposed from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Furthermore, the release of a little amount of CO decomposed from functional groups might decrease the content of peak 1 as well. M. A. Nahil and P. T. Williams suggested that in the range of 150–400 °C the pyrolysis of organic matter in the cotton stalks and dehydration of phosphoric acid were shown in the thermal degradation.41 BTRHR in our study was different from the original biomass waste because of the bio-oil extraction from rice husk and then the base treatment. Therefore, the composition might have already changed, and the weight loss should be mainly attributed to the dehydration of phosphoric acid and the decomposition of some functional groups in this stage.
O group. Furthermore, the release of a little amount of CO decomposed from functional groups might decrease the content of peak 1 as well. M. A. Nahil and P. T. Williams suggested that in the range of 150–400 °C the pyrolysis of organic matter in the cotton stalks and dehydration of phosphoric acid were shown in the thermal degradation.41 BTRHR in our study was different from the original biomass waste because of the bio-oil extraction from rice husk and then the base treatment. Therefore, the composition might have already changed, and the weight loss should be mainly attributed to the dehydration of phosphoric acid and the decomposition of some functional groups in this stage.
From 400 to 700 °C, Hn+2PnO3n+1 should dehydrate and transform into P4O10, which presents various crystalline polymorphs and a vitreous modification, two such polymorphs melt between 500 and 600 °C.42 P4O10 which behaved as an oxidant reacted with carbon, forming new pores, widening the existing pores and producing CO2. The phosphorus compound product of the reaction between P4O10 and carbon was not phosphorus from the XPS of P 2p. It might be P4O6 according to O 1s and P 2p. The following reactions might occur:
| Hn+2PnO3n+1 → P4O10 + H2O | (4) |
| P4O10 + C → P4O6 + CO2 | (5) |
The content of peak 1 decreased obviously from Table 5. It is mainly due to reduction of P4O10 to P4O6, leading to the decrease of the content of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The release of H2O and CO2 in this temperature range could be confirmed in TPD-MS. A little amount of CO decomposed from functional groups also existed in this stage.
O. The release of H2O and CO2 in this temperature range could be confirmed in TPD-MS. A little amount of CO decomposed from functional groups also existed in this stage.
From 700 to 800 °C, P4O10 and/or P4O6 might react with CHx in the substrate, generating PH3 which could be detected in TPD-MS. The following reaction might occur:
| P4O10/P4O6 + CHx → PH3 + CO2/CO | (6) |
The amount of CO2 and CO generated in this temperature range showed sharp increase. Moreover, the reaction between CO2 and carbon to produce CO might exist at the same time. A. M. Puziy et al. found that elemental phosphorus was observed in the matrix of polymer-based carbon at the temperature as high as 1000 °C.38 M. Myglovets et al. who take high-molecular-weight softwood sodium lignosulfonate as precursor pointed out that volatile phosphorus compounds might be formed by the following reactions above 750 °C:43
| 4H3PO4 + 10C → P4 + 10CO + 6H2O |
| 4H3PO4 + 10C → P4O10 + 6H2O |
| P4O10 + 10C → P4 + 10CO |
The hydrogen elemental composition of BTRHR was low in our study, however, PH3 still generated above 700 °C. The variation of phosphorus differed from these studies. This might be due to the difference between the precursors.
In our study, the activation process was divided in three temperature zones according to TGA-DTG results: the release of absorbed water and dehydration of H3PO4, the release of CO2 and CO from the decomposition of functional groups from 100 to 400 °C; the release of H2O dehydrated from H3PO4, the release of CO from the decomposition of functional groups, and CO2 decomposed from the reaction and functional groups in the temperature range of 400–700 °C; PH3 generated from the reaction, the release of CO2 and CO generated from the reactions and the decomposition of functional groups from 700 to 800 °C. Above all, phosphoric acid played an important role in the activation process: (i) phosphoric acid seemed to be able to function as an acid catalyst in promoting bond cleavage reactions and the formation of crosslinks via such processes as cyclization and condensation; (ii) phosphoric acid acted as an oxidant after dehydration; and (iii) phosphoric acid connected with the substrate entering AC through C–O–P bond.
4. Conclusions
A series of AC samples were prepared from RHR by base treatment and chemical activation process in this study. The base treatment could remove ash and improve the surface area and pore volume. Chemical activation played an important role in increasing the surface area and pore volume. The valence state of phosphorus changed from +5 to −3 during the chemical activation process with H3PO4 from 100 to 800 °C. The reaction processes is quite complicated. The best results in terms of surface area (1016 m2 g−1) was observed for a BTRHR/H3PO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, an activation temperature of 500 °C and an activation time of 1 h.
2, an activation temperature of 500 °C and an activation time of 1 h.
Acknowledgements
This research was financially supported by the National High Technology Research and Development Program (863 program 2012 AA 051803) of China and the characterization of the samples from Analytic and Testing Center of Sichuan University was highly acknowledged.Notes and references
- X. Song, Y. Zhang and C. Chang, Ind. Eng. Chem. Res., 2012, 51, 15075–15081 CrossRef CAS.
- W. Shi, J. Jia, Y. Gao and Y. Zhao, Bioresour. Technol., 2013, 146, 355–362 CrossRef CAS PubMed.
- J. Alvarez, G. Lopez, M. Amutio, J. Bilbao and M. Olazar, Fuel, 2014, 128, 162–169 CrossRef CAS PubMed.
- X. Zhang, Y. Li, G. Li and C. Hu, RSC Adv., 2015, 5, 4984–4992 RSC.
- Y. Liu, Y. Guo, Y. Zhu, D. An, W. Gao, Z. Wang, Y. Ma and Z. Wang, J. Hazard. Mater., 2011, 186, 1314–1319 CrossRef CAS PubMed.
- Y. Liu, Y. Guo, W. Gao, Z. Wang, Y. Ma and Z. Wang, J. Cleaner Prod., 2012, 32, 204–209 CrossRef CAS PubMed.
- T. H. Liou and S. J. Wu, J. Hazard. Mater., 2009, 171, 693–703 CrossRef CAS PubMed.
- O. A. Ioannidou, A. A. Zabaniotou, G. G. Stavropoulos, M. A. Islam and T. A. Albanis, Chemosphere, 2010, 80, 1328–1336 CrossRef CAS PubMed.
- M. Molina-Sabio and F. Rodríguez-Reinoso, Colloids Surf., A, 2004, 241, 15–25 CrossRef CAS PubMed.
- N. V. Sych, S. I. Trofymenko, O. I. Poddubnaya, M. M. Tsyba, V. I. Sapsay, D. O. Klymchuk and A. M. Puziy, Appl. Surf. Sci., 2012, 261, 75–82 CrossRef CAS PubMed.
- V. Fierro, G. Muñiz, A. H. Basta, H. El-Saied and A. Celzard, J. Hazard. Mater., 2010, 181, 27–34 CrossRef CAS PubMed.
- M. Al Bahri, L. Calvo, M. A. Gilarranz and J. J. Rodriguez, Chem. Eng. J., 2012, 203, 348–356 CrossRef CAS PubMed.
- L. Lin, S. R. Zhai, Z. Y. Xiao, Y. Song, Q. D. An and X. W. Song, Bioresour. Technol., 2013, 136, 437–443 CrossRef CAS PubMed.
- C. Michailof, G. G. Stavropoulos and C. Panayiotou, Bioresour. Technol., 2008, 99, 6400–6408 CrossRef CAS PubMed.
- M. Jagtoyen and F. Derbyshire, Carbon, 1998, 36, 1085–1097 CrossRef CAS.
- J. M. Rosas, R. Ruiz-Rosas, J. Rodríguez-Mirasol and T. Cordero, Carbon, 2012, 50, 1523–1537 CrossRef CAS PubMed.
- Q. Lu, X.-L. Yang and X.-F. Zhu, J. Anal. Appl. Pyrolysis, 2008, 82, 191–198 CrossRef CAS PubMed.
- I. Othman Ali, A. M. Hassan, S. M. Shaaban and K. S. Soliman, Sep. Purif. Technol., 2011, 83, 38–44 CrossRef PubMed.
- L. J. Kennedy, J. J. Vijaya and G. Sekaran, Ind. Eng. Chem. Res., 2004, 43, 1832–1838 CrossRef CAS.
- P. Barpanda, G. Fanchini and G. G. Amatucci, Carbon, 2011, 49, 2538–2548 CrossRef CAS PubMed.
- Y. Guo and D. A. Rockstraw, Microporous Mesoporous Mater., 2007, 100, 12–19 CrossRef CAS PubMed.
- J. Zhang, H. Fu, X. Lv, J. Tang and X. Xu, Biomass Bioenergy, 2011, 35, 464–472 CrossRef CAS PubMed.
- Y. Juan and Q. Ke-Qiang, Environ. Sci. Technol., 2009, 43, 3385–3390 CrossRef.
- T.-H. Liou, Carbon, 2004, 42, 785–794 CrossRef CAS PubMed.
- A. M. Puziy, O. I. Poddubnaya, A. Martıínez-Alonso, F. Suárez-García and J. M. D. Tascón, Carbon, 2002, 40, 1493–1505 CrossRef CAS.
- Y. Chen, S. R. Zhai, N. Liu, Y. Song, Q. D. An and X. W. Song, Bioresour. Technol., 2013, 144, 401–409 CrossRef CAS PubMed.
- R. Pierotti and J. Rouquerol, Pure Appl. Chem., 1985, 57, 603–619 Search PubMed.
- H. Teng, T.-S. Yeh and L.-Y. Hsu, Carbon, 1998, 36, 1387–1395 CrossRef CAS.
- M. Molina-Sabio, F. Rodriguez-Reinoso, F. Caturla and M. Selles, Carbon, 1995, 33, 1105–1113 CrossRef CAS.
- S. Timur, I. C. Kantarli, E. Ikizoglu and J. Yanik, Energy Fuels, 2006, 20, 2636–2641 CrossRef CAS.
- Y. Li, X. Ding, Y. Guo, C. Rong, L. Wang, Y. Qu, X. Ma and Z. Wang, J. Hazard. Mater., 2011, 186, 2151–2156 CrossRef CAS PubMed.
- D. Prahas, Y. Kartika, N. Indraswati and S. Ismadji, Chem. Eng. J., 2008, 140, 32–42 CrossRef CAS PubMed.
- M. Zhi, F. Yang, F. Meng, M. Li, A. Manivannan and N. Wu, ACS Sustainable Chem. Eng., 2014, 2, 1592–1598 CrossRef CAS.
- L. Ding, B. Zou, L. Shen, C. Zhao, Z. Wang, Y. Guo, X. Wang and Y. Liu, Colloids Surf., A, 2014, 446, 90–96 CrossRef CAS PubMed.
- T. Durkić, A. Perić, M. Laušević, A. Dekanski, O. Nešković, M. Veljković and Z. Laušević, Carbon, 1997, 35, 1567–1572 CrossRef.
- M. O. Guerrero-Pérez, M. J. Valero-Romero, S. Hernández, J. M. L. Nieto, J. Rodríguez-Mirasol and T. Cordero, Catal. Today, 2012, 195, 155–161 CrossRef PubMed.
- P. Y. Shih, S. W. Yung and T. S. Chin, J. Non-Cryst. Solids, 1999, 244, 211–222 CrossRef CAS.
- A. M. Puziy, O. I. Poddubnaya, R. P. Socha, J. Gurgul and M. Wisniewski, Carbon, 2008, 46, 2113–2123 CrossRef CAS PubMed.
- A. Castro-Muniz, F. Suarez-Garcia, A. Martinez-Alonso and J. M. Tascon, J. Colloid Interface Sci., 2011, 361, 307–315 CrossRef CAS PubMed.
- M. Zhi, S. Liu, Z. Hong and N. Wu, RSC Adv., 2014, 4, 43619–43623 RSC.
- M. A. Nahil and P. T. Williams, Biomass Bioenergy, 2012, 37, 142–149 CrossRef CAS PubMed.
- M. Olivares-Marín, C. Fernández-González, A. Macías-García and V. Gómez-Serrano, Carbon, 2006, 44, 2347–2350 CrossRef PubMed.
- M. Myglovets, O. I. Poddubnaya, O. Sevastyanova, M. E. Lindström, B. Gawdzik, M. Sobiesiak, M. M. Tsyba, V. I. Sapsay, D. O. Klymchuk and A. M. Puziy, Carbon, 2014, 80, 771–783 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |