Effect of end-groups on simultaneous oleophobicity/hydrophilicity and anti-fogging performance of nanometer-thick perfluoropolyethers (PFPEs)†
Yongjin Wang,
James Knapp,
Aleigh Legere,
Jacob Raney and
Lei Li*
Department of Chemical and Petroleum Engineering, University of Pittsburgh, Pittsburgh, PA 15261, USA. E-mail: lel55@pitt.edu
First published on 25th March 2015
Abstract
Simultaneously oleophobic/hydrophilic coatings are highly desirable in many important applications, e.g., anti-fogging. However, to date, such coatings have been rarely reported and the underlying mechanisms remain unclear. In the current paper, the wetting behavior of three nanometer-thick perfluoropolyether (PFPE) polymers with the same backbone but different end-groups has been studied by contact angle tests and the underlying mechanisms governing the simultaneous oleophobicity/hydrophilicity have been investigated. The experimental results indicated that the end-groups of the nanometer-thick PFPEs are critical to the simultaneous oleophobicity/hydrophilicity. PFPE polymers with different end-groups can interact with the substrate in very different ways, resulting in different packing orders and thus different inter-chain distances within the polymer nanofilms. If the inter-chain distance is appropriately small, smaller water molecules penetrate the nanofilms quickly while larger oil molecules penetrate the nanofilms much more slowly. As a result, the surface shows a higher oil contact angle (OCA) than WCA, i.e., simultaneous oleophobicity/hydrophilicity. Moreover, the effect of simultaneous oleophobicity/hydrophilicity on the long-term anti-fogging capability has been studied by X-ray photoelectron spectroscopy (XPS) and anti-fogging tests. The results indicated that the unique simultaneous oleophobicity/hydrophilicity reduces the airborne hydrocarbon contamination and therefore improves the long-term anti-fogging performance.
1. Introduction
Simultaneously oleophobic/hydrophilic coatings are highly desirable in many important applications, e.g., anti-fogging. Fogging, which often occurs on the surface of glasses, goggles, camera lenses and automobile windows, reduces the light transmission and could degrade the device performance dramatically.1 To understand the fogging mechanism, Briscoe1 studied the condensation of water vapor on the surfaces of polyethylene and glass and found that when the water contact angle (WCA) on a solid surface is low, a uniform water film will be formed and fogging will not occur. When WCA is higher than a critical value, water droplets will be formed on the solid surface and fogging will occur and reduce the light transmission. In recent years, different anti-fogging coatings have been developed in order to improve the effectiveness of the optical devices,2–6 including multilayer nanoporous-silicate,3–5 TiO2-based nanoparticle coatings6 and solvent-sensitive stimuli-responsive polymer brush coatings.2 All of these anti-fogging coatings are designed to be hydrophilic and it is expected that water will form a uniform film on the coated surface and, as a result, fogging will not occur. Indeed, this is the case in the short term. Unfortunately, in long-term applications these hydrophilic coatings, which have high surface energy, are easily contaminated by airborne hydrocarbons in the ambient environment.2 The adsorbed airborne hydrocarbons on the surface will lower the surface energy and thus render the surface more hydrophobic. As a result, water forms droplets on such a “oil-coated” surface, which makes the surface no longer anti-fogging.2,7,8 A promising approach to address this issue is to develop simultaneously oleophobic/hydrophilic coatings, which are expected to allow water to easily wet the surface due to their “hydrophilicity” and reduce the hydrocarbon contamination due to their “oleophobicity”. Unfortunately, WCA is usually higher than oil contact angle (OCA) on the same solid surface according to previous theoretical and experimental studies.9,10 The lower OCA indicates that a solid is expected to be more wettable to oil than to water. In other words, if a solid surface is hydrophilic, it cannot be oleophobic. However, a few recent reports indicated that it is possible to achieve simultaneous oleophobicity/hydrophilicity with some nanometer-thick amphiphilic coatings on some hydrophilic substrates.7,8,11,12 For example, polyelectrolyte plasma polymer substrates, coated with ionic amphiphilic fluoro-surfactants, were reported to be more wettable to water than to oil.8,11 Moreover, a reactive isocyanate-silane modified silica substrate, covalently grafted with perfluorinated polyethylene glycol oligomers (f-PEG) layers, was reported to have significantly lower WCA than hexadecane contact angle (HCA).7,12 The possible mechanisms of simultaneous oleophobicity/hydrophilicity have been discussed previously.7,8,11,12 Howarter and Youngblood7,12 proposed that the PEG segments, which is hydrophilic, stay on the bottom of the polymer layer and the perfluorinated segments, which is hydrophobic, stay on the top of the layer. Water would swell the PEG constituents while hexadecane wouldn't as it is not a good solvent for PEG. Therefore water would “see” the hydrophilic PEG segments, resulting in a small WCA, and hexadecane would “see” the hydrophobic perfluorinated segments, resulting in a larger HCA. In other words, they proposed that water and hexadecane “see” two different “surface energies” on the same surface. However, a given solid surface should have only one fixed surface energy. Clearly, further research is required to uncover the underlying mechanisms of the simultaneously oleophobic/hydrophilic coatings.In the current paper, based on our recent finding13 that a nanometer-thick PFPE with hydroxyl end-groups shows simultaneous oleophobicity/hydrophilicity on a silica substrate, we have investigated the mechanisms of simultaneous oleophobicity/hydrophilicity by systematically varying the end-groups of PFPEs. The static and time-dependent WCA and HCA results suggested that the simultaneous oleophobicity/hydrophilicity can be achieved only when the end-groups of PFPEs have the appropriate interaction with the substrate so that the inter-chain distance of PFPE in the nanofilm is appropriately small. In this case, water penetrates the polymer nanofilm quickly while oil penetrates the nanofilm much more slowly. As a result, the coating shows simultaneous oleophobicity/hydrophilicity. More interestingly, X-ray photoelectron spectroscopy (XPS) and anti-fogging testing results showed that the simultaneously oleophobic/hydrophilic coating is indeed more resistant to airborne hydrocarbon contamination, leading to improved long-term anti-fogging performance.
2. Experimental
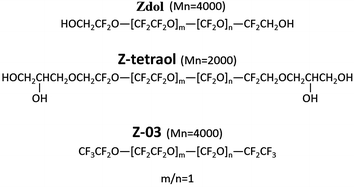
Si wafer (purchased from Silicon Quest International, Inc.) with 2 nm native oxide (P/B <100> 1–10 ohm cm, 279 ± 25 μm) and plain glass microscope slides (25 × 7 × 51 mm, Fisherbrand) were used as the substrates for contact angle and anti-fogging tests, respectively. The substrates were coated with three PFPE polymers, commercially known as Zdol, Z-03 and Z-tetraol, which were obtained from Solvay Solexis Inc. The chemical structures of PFPEs are shown in Fig. 1.These three PFPEs have the same backbone but different end-groups, which result in the different interactions with silica surfaces.14–16 The nanofilms were coated on silica wafer by dip-coating13 with 2,3-dihydrodecafluoropentaneas as the solvent, which was obtained from Miller Stephenson Chemical Corporation. Deionized (DI) water, produced from a Millipore Academic A10 system with total organic carbon below 40 ppb, and hexadecane (anhydrous, ≥99%, Sigma-Aldrich) were used as the testing liquids for contact angle measurements. All of the chemicals were utilized as received.
The chemical structure of PFPE nanofilms was characterized using ESCALAB 250 XI (Thermo Scientific) X-ray photoelectron spectroscopy (XPS) system. The spectra were collected using a monochromatic Al Kα X-ray source with a spot size of 200 μm. An electron flood gun was used to neutralize charge on the non-conductive samples. For each experiment, the sample was inserted into the analysis chamber where a vacuum was maintained at ∼10 × 10−10 mBar by magnetic manipulator. Then a survey spectrum (5 scans, 150 eV pass energy, 1 eV step) and a high-resolution C1s scan (20 scans, 50 eV pass energy, 0.1 eV step) were collected. The spectra analysis was performed using Thermo Avantage software.
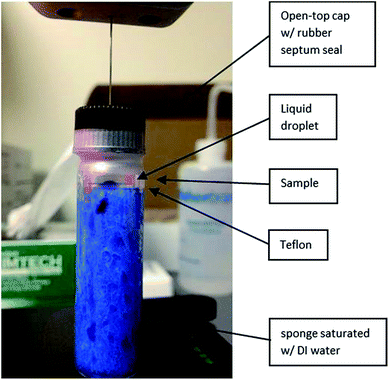
The WCA and HCA on the PFPE-coated Si wafers were measured using a VCA optima XE (AST Production Inc.) video contact angle system. For the static contact angle test, a 2 μL liquid droplet was deposited on the sample surface and then the image of this liquid droplet on the surface was taken with a charge-coupled device (CCD) camera and eventually the contact angle was determined by the VCA software automatically. To minimize the possible interruption caused by the evaporation of the liquid, the time-dependent WCA test was done in a sealed system and the sample was left on the stage of the goniometer during the entire testing period. The sealed system is shown in Fig. 2: a 4.5 cm × 1 cm × 1 cm section of sponge was placed in a round glass cuvette. The sponge was soaked with the testing liquid, e.g. water. An open-top septum cap with a rubber seal was utilized for the cuvette and the parafilm was used to further seal the system. Since the needle of VCA instrument penetrates the rubber septum easily, testing liquids can be introduced on the sample without opening the cuvette. After placing the droplet of the testing liquid on each sample, contact angles of the same droplet were monitored in the following 24 hours.
To characterize the anti-fogging performance of the nanometer-thick polymer coatings, the PFPE-coated glass slides were held over the boiling water for five seconds and then removed and photographed.7
3. Results
3.1 Static and time-dependent contact angle tests
The static WCA and HCA on three PFPE-coated Si wafers are shown in Table 1, which indicate totally different wetting behavior. The WCA on Z-03/Si is 43.4°, which is larger than the HCA (32.6°), and thus Z-03/Si still shows “normal” wetting behavior: WCA is larger than HCA. On the contrary, Zdol/Si is simultaneously oleophobic/hydrophilic as WCA (46.5°) is lower than HCA (70.1°). Interestingly, for Z-tetraol/Si, both WCA (66.2°) and HCA (68.8°) are relatively large and they have similar values.| DI Water | Hexadecane | |
|---|---|---|
| Z-03/Si | 43.4 ± 0.8 | 32.6 ± 0.5 |
| Zdol/Si | 46.5 ± 0.9 | 70.1 ± 0.9 |
| Z-tetraol/Si | 66.2 ± 1.2 | 68.8 ± 1.2 |
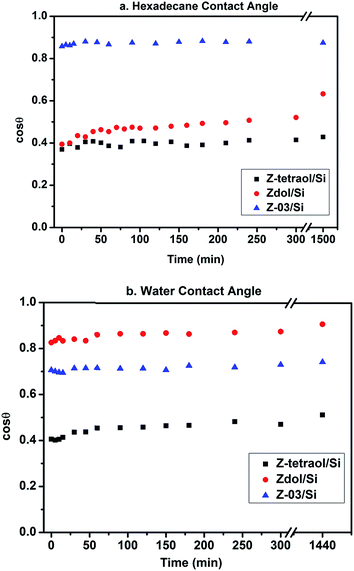
The time-dependent HCA and WCA on three PFPE-coated Si wafers are shown in Fig. 3a and b, respectively. As shown in Fig. 3a, HCA does not change with time up to 1500 min for both Z-tetraol/Si and Z-03/Si. However, for Zdol/Si, cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (HCA) changes from 0.39 (66.8°) to 0.63 (50.8°) during the same time period. This is consistent with previous13,17 reports that the HCA on Zdol/Si decreases with time. Since hexadecane does not evaporate during the experiment,13 the change in HCA cannot be attributed to the evaporation as reported before.18 Instead, the results indicate the “penetration” of hexadecane through the Zdol nanofilms10 (more detailed discussion on this is provided in ESI†). As shown in Fig. 3b, all three samples show similar trend of time-dependent WCA: cos
θ (HCA) changes from 0.39 (66.8°) to 0.63 (50.8°) during the same time period. This is consistent with previous13,17 reports that the HCA on Zdol/Si decreases with time. Since hexadecane does not evaporate during the experiment,13 the change in HCA cannot be attributed to the evaporation as reported before.18 Instead, the results indicate the “penetration” of hexadecane through the Zdol nanofilms10 (more detailed discussion on this is provided in ESI†). As shown in Fig. 3b, all three samples show similar trend of time-dependent WCA: cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ doesn't change significantly during 24 hours (the slight change of WCA can be attributed to the evaporation effect and more detailed discussion is provided in ESI†).
θ doesn't change significantly during 24 hours (the slight change of WCA can be attributed to the evaporation effect and more detailed discussion is provided in ESI†).
3.2 XPS and anti-fogging tests
The anti-fogging test results of PFPE-coated glass slides are shown in Fig. 4. The tests were conducted on the 1st day and the 14th day after the samples were fabricated. Z-tetraol showed the worst anti-fogging performance on both days and this can be attributed to the fact that it is the most hydrophobic one with the highest WCA among three PFPEs. Zdol and Z-03 showed similarly good anti-fogging performance on the 1st day. However, with the increase of aging time, the anti-fogging performance of Z-03 degraded significantly while there was much less change for Zdol. As a result, on the 14th day, Zdol showed significantly better anti-fogging performance than Z-03.To understand the relationship between airborne hydrocarbon contamination and the long-term antifogging performance, the XPS C1s spectra of Zdol, Z-tetraol and Z-03 coated glass slides have been collected and are shown in Fig. 5. To synchronize with anti-fogging tests, the XPS experiments were also conducted on the 1st day and the 14th day after the samples were fabricated, respectively. The peaks at 295.12 eV and 293.03 eV are assigned to fluorocarbon constituents and the peaks between 282.00 to 290.00 eV are assigned to hydrocarbon constituents based on previous literature.19 Since there is no hydrocarbon moiety in Z-03 molecule, all the hydrocarbon peaks are attributed to the airborne hydrocarbon contaminants for Z-03 sample. Because both Zdol and Z-tetraol polymers contain hydrocarbon moiety in their end-groups, the hydrocarbon peaks are attributed to both airborne hydrocarbon contaminants and the end-groups of polymers (see ESI† for the detailed calculation procedure).
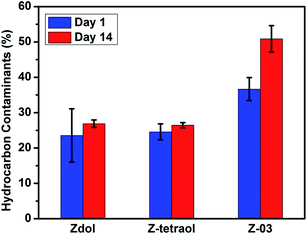
The amount of airborne hydrocarbon contaminants (in atomic% of total carbon; mean value of the three repeats) at day 1 and day 14 are shown in Fig. 6. On the 1st day after the samples were fabricated, the amount of airborne hydrocarbon contaminants was 23.56%, 24.56% and 36.68% for Zdol, Z-tetraol and Z-03, respectively. And on the 14th day, the amount of airborne hydrocarbon contaminants was 26.89%, 26.46% and 50.88% for Zdol, Z-tetraol and Z-03, respectively. Between the 1st day and the 14th day, the amount of airborne hydrocarbon contaminants increased by 14.2%, 2.9% and 1.9% for Z-03, Zdol and Z-tetraol, respectively. Clearly, Z-03 is the most easily contaminated by the airborne hydrocarbons.
4. Discussions
4.1 Underlying mechanisms of simultaneous oleophobicity/hydrophilicity
As shown in Table 1, three PFPE-coated silica surfaces show different wetting behaviors: Zdol/Si shows a characteristic of simultaneous oleophobicity/hydrophilicity; Z-tetraol/Si is both “hydrophobic” and “oleophobic” while Z-03/Si shows the “normal” wetting behavior with WCA higher than HCA. Since the only differences among three PFPEs are molecular weights and end-groups and the molecular weight has little effect on the wetting behavior (see ESI† for more details). Therefore, the end-groups are the key to the simultaneous oleophobicity/hydrophilicity. Chen et al.20 studied the interaction between PFPE polymers and hydrophilic substrates and suggested that Zdol molecules form hydrogen bonding with hydrophilic substrates via the hydroxyl end-groups, leading to an ordered packing structure of polymer chains. If this is true, Z-03 molecules should have a disordered packing structure on the silicon wafer since Z-03 doesn't have hydroxyl end-groups and thus cannot form hydrogen bonds with hydrophilic substrates. Moreover, since Z-tetraol has more hydroxyls end-groups than Zdol,21 Z-tetraol molecules are expected to have stronger attraction to the silicon wafer, resulting in a highly ordered packing structure.22 According to the discussion above, a proposed “bonding” model for PFPE polymers and hydrophilic substrates is schematically shown in Fig. 7. Both Zdol and Z-tetraol form hydrogen bonds with silica surfaces and Z-tetraol has a more ordered packing structure than Zdol. Z-03 doesn't form hydrogen bonds with silica surface, leading to a disordered packing structrue.Based on this model, different packing structure will lead to different inter-chain distances, i.e., the size of the intermolecular “holes”. The highly ordered Z-tetraol nanofilm should have the smallest intermolecular “holes”, i.e., inter-chain distance, and the disordered Z-03 nanofilm should have the largest intermolecular “holes”. When a liquid is placed on PFPE-coated silica surface, it has the tendency to penetrate the nanofilm to “see” the substrate instead of fluorinated surface since the substrate has higher surface energy.13,23,24 The penetration rate is dependent on both the molecular size of the liquid and the size of the intermolecular “holes” within the polymer nanofilm. If the molecular size of the liquid is smaller than the size of the “holes”, it will penetrate the nanofilm quickly and reach an equilibrium contact angle value on the substrate in a short time period. If the molecular size of the liquid is much larger than the size of the “holes”, it will penetrate the nanofilm much more slowly. For Z-03, the size of the intermolecular “holes” is large so that both water and hexadecane penetrate the nanofilm quickly, resulting in a normal wetting behavior with WCA larger than HCA. For Z-tetraol, the size of the intermolecular “holes” is so small that both water and hexadecane cannot penetrate the nanofilm. As a result, both HCA and WCA are relatively high. For Zdol, the size of the intermolecular “holes” is appropriately small so that water molecules penetrate the nanofilm quickly while hexadecane molecules penetrate the nanofilm much more slowly, which renders HCA higher than WCA.
The above-mentioned mechanisms are further supported by the time-dependent contact angle results. As shown in Fig. 3, the HCA significantly decreases with time only for Zdol/Si and doesn't change with time for Z-03/Si and Z-tetraol/Si. The time-dependent HCA on Zdol/Si cannot be explained by the rearrangement of the polymer segments. Thermodynamically, the fluorinated backbone of Zdol polymer will stay on the top of the nanofilms and the hydroxyl end-groups will stay on the bottom of the nanofilms to minimize the overall interfacial energy for Zdol/Si sample.13 After a drop of hexadecane is placed on the surface, the only possible segmental rearrangement is that the hydroxyl end-groups move to the top of the nanofilms. However, since the hydroxyl end-groups “like” the hydrophilic silica substrate more than the hexadecane, there is no driving force for this segmental rearrangement to occur. Moreover, if this segmental rearrangement indeed occurred, we should have observed similar time-dependent HCA for Z-tetraol/Si because Z-tetraol also has hydroxyl end-groups. However, experimental results show that HCA on Z-tetraol/Si does not change with time. Therefore, the observed time-dependent HCA on Zdol/Si cannot be attributed to the segmental rearrangement. Instead, this can be reasonably explained by the different packing orders and the resulting inter-chain distances of three PFPEs. The inter-chain distance in Z-03 is large so that the “penetration” of hexadecane occurs instaneously. As a result, no time-dependence of HCA was observed for Z-03 sample. For Z-tetraol, the inter-chain distance is so small that no “penetration” can occur and thus no time-dependence of HCA was observed for Z-tetraol sample, either. For Zdol, the inter-chain distance is appropriately small so that hexadecane molecules penetrate the nanofilm slowly and show clear time-dependence. In contrast, there is no time dependence in WCA for all three samples as shown in Fig. 3. This can be attributed to the fact that the “penetration” of water molecule occurs instantaneously for Zdol and Z-03 coatings while no “penetration” of water molecule occurs for Z-tetraol coating.
To summarize, as shown in Fig. 8, a model has been proposed to describe the “penetration” mechanisms of hexadecane and water at the Zdol/Si interface, which results in the observed simultaneous oleophobicity/hydrophilicity. After a drop of hexadecane is placed on the Zdol/Si surface, initially hexadecane molecules “see” a fluorinated surface and therefore show a high HCA in a short time period. Gradually, HCA decreases as the “penetration” proceeds. On the contrary, smaller water penetrates the nanofilm very quickly and reaches the equilibrium value on the substrate almost immediately after a drop of water is placed on the Zdol/Si surface. As a result, immediately after the contact angle measurement, the static WCA is close to the equilibrium value while the HCA is much higher than the equilibrium value. Therefore, the observed higher HCA (than WCA) is kinetically controlled rather than thermodynamically determined.
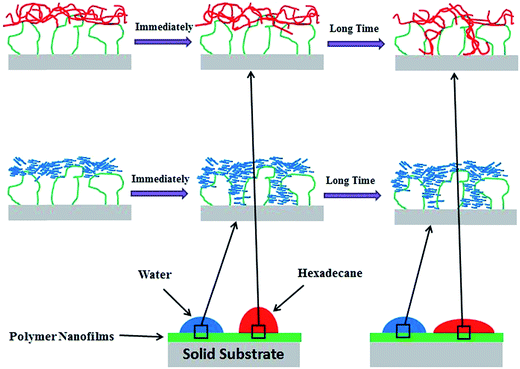 | ||
| Fig. 8 Schematic show of the “penetration” mechanism of water and hexadecane on Zdol/Si (not shown to scale). | ||
4.2 Airborne hydrocarbon contamination and anti-fogging performance
When a cold glass slide meets humid air, water will condense on the glass surface, which could reduce the transmittance of the glass slide.25 If the surface is hydrophilic, a water film will be formed on the surface and fogging will not occur. If the surface is hydrophobic, water droplets will be formed on the surface and fogging will occur.1 For the three PFPE samples, Z-tetraol is the most hydrophobic one and thus it exhibits the worst anti-fogging performance, regardless of aging time, as shown in Fig. 4. On the contrary, the anti-fogging performance of Z-03 and Zdol samples is good right after sample fabrication and degrades with the aging time. Rangel et al. reported that a solid surface exposed to the ambient environment could be contaminated due to the adsorption of the airborne hydrocarbons.26,27 For the surface with “normal” wetting behavior, OCA is lower than WCA and thus the hydrocarbon contamination will occur easily. As a result, the originally hydrophilic surface becomes more hydrophobic and not anti-fogging anymore. However, for the surfaces with simultaneously oleophobic/hydrophilic behavior, hydrocarbon contamination is expected to be reduced due to the oleophobicity and the long-term anti-fogging performance should be improved. Indeed, Youngblood and Howarter showed that simultaneously oleophobic/hydrophilic f-PEG is promising for anti-fogging coating.7 More recently, Badyal and et al. reported that the solvent-cast copolymer–fluorosurfactant complexes, which show simultaneously oleophobic/hydrophilic behavior, also have excellent anti-fogging performance.28 However, there has not been reported whether or not the anti-fogging performance will change with the aging time. Moreover, the relationship between hydrocarbon contamination and the anti-fogging performance has not yet been experimentally demonstrated. The anti-fogging testing and XPS results shown in Fig. 4 and 5, respectively, shed the new light to these questions. On the 1st day, both Zdol and Z-03 have the least airborne hydrocarbon contaminants and show good anti-fogging performance. On the 14th day, Z-03 has significantly more airborne hydrocarbon contaminants than Zdol and also shows significantly worse anti-fogging performance than Zdol, which is simultaneously oleophobic/hydrophilic. These results indicate that the simultaneously oleophobic/hydrophilic surfaces do slow down the airborne hydrocarbon contamination and therefore have improved long-term anti-fogging performance (Fig. 4 and 5 show that Zdol and Z-03 have similarly good anti-fogging performance while Z-03 has more airborne hydrocarbon contaminants than Zdol at day 1. This can be attributed to the fact that the anti-fogging experiments were performed immediately after the samples were fabricated, where both Zdol and Z-03 were rarely polluted by the airborne hydrocarbon contaminants. However, the XPS experiments were conducted several hours after the samples were fabricated and it is likely that more airborne hydrocarbon contaminants were adsorbed on Z-03 than on Zdol during the time period). Moreover, since PFPE Zdol is an excellent lubricant, which has been utilized in hard disk drive industry and aerospace application,29 and has very good high-temperature stability,29 it will provide outstanding long-term reliability in the real-life anti-fogging applications.5. Conclusions
Both static and time-dependent WCA and HCA results suggested that PFPE polymers with different end-groups interact with the hydrophilic substrates in very different ways, leading to different packing order of polymer chains and thus different inter-chain distances, i.e., the size of the intermolecular “holes”. Only when the size of the intermolecular “holes” is appropriately small, the large oil molecules penetrate the polymer layer slowly while the small water molecules penetrate the polymer layer quickly, resulting in a larger OCA than WCA in a short time period, i.e., simultaneous oleophobicity/hydrophilicity. The XPS and anti-fogging test results demonstrated that simultaneously oleophobic/hydrophilic surfaces effectively reduce the airborne hydrocarbon contamination and therefore have an improved long-term anti-fogging performance.Acknowledgements
Y.W. and L.L. gratefully acknowledge the financial support from NSF (CMMI-1233161). We also thank Dr Michael Stirniman and Dr Jiping Yang from Seagate Technology LLC for providing the PFPE samples.References
- B. Briscoe and K. Galvin, Solar Energy, 1991, 46, 191–197 CrossRef.
- J. A. Howarter and J. P. Youngblood, Adv. Mater., 2007, 19, 3838–3843 CrossRef CAS.
- H. Shimomura, Z. Gemici, R. E. Cohen and M. F. Rubner, ACS Appl. Mater. Interfaces, 2010, 2, 813–820 CAS.
- L. Zhang, Y. Li, J. Sun and J. Shen, Langmuir, 2008, 24, 10851–10857 CrossRef CAS PubMed.
- F. Ç. Cebeci, Z. Wu, L. Zhai, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 2856–2862 CrossRef CAS PubMed.
- Y. Lai, Y. Tang, J. Gong, D. Gong, L. Chi, C. Lin and Z. Chen, J. Mater. Chem., 2012, 22, 7420–7426 RSC.
- J. A. Howarter and J. P. Youngblood, Macromol. Rapid Commun., 2008, 29, 455–466 CrossRef CAS.
- R. Lampitt, J. Crowther and J. Badyal, J. Phys. Chem. B, 2000, 104, 10329–10331 CrossRef CAS.
- E. G. Shafrin and W. A. Zisman, Upper limits for the contact angle of liquids on solides, DTIC Document, 1963 Search PubMed.
- C. Extrand and Y. Kumagai, J. Colloid Interface Sci., 1997, 191, 378–383 CrossRef CAS.
- S. Hutton, J. Crowther and J. Badyal, Chem. Mater., 2000, 12, 2282–2286 CrossRef CAS.
- J. A. Howarter and J. P. Youngblood, Adv. Mater., 2007, 19, 3838–3843 CrossRef CAS.
- L. Li, Y. Wang, C. Gallaschun, T. Risch and J. Sun, J. Mater. Chem., 2012, 22(33), 16719–16722 RSC.
- R. Waltman, G. Tyndall and J. Pacansky, Langmuir, 1999, 15, 6470–6483 CrossRef CAS.
- G. Tyndall, R. Waltman and D. Pocker, Langmuir, 1998, 14, 7527–7536 CrossRef CAS.
- A. Merzlikine, L. Li, P. M. Jones and Y.-T. Hsia, Tribol. Lett., 2005, 18, 279–286 CrossRef CAS PubMed.
- J. Wu and C. M. Mate, Langmuir, 1998, 14, 4929–4934 CrossRef CAS.
- C. Bourges-Monnier and M. Shanahan, Langmuir, 1995, 11, 2820–2829 CrossRef CAS.
- C. M. Mate and V. Novotny, J. Chem. Phys., 1991, 94, 8420–8427 CrossRef CAS PubMed.
- H. Chen, L. Li, A. G. Merzlikine, Y.-T. Hsia and M. S. Jhon, J. Appl. Phys., 2006, 99, 08N103 Search PubMed.
- B. Bhushan and Z. Tao, Microsyst. Technol., 2006, 12, 579–587 CrossRef CAS PubMed.
- Z. Tao and B. Bhushan, Wear, 2005, 259, 1352–1361 CrossRef CAS PubMed.
- H. Tavana and A. Neumann, Adv. Colloid Interface Sci., 2007, 132, 1–32 CrossRef CAS PubMed.
- C. Lam, N. Kim, D. Hui, D. Kwok, M. Hair and A. Neumann, Colloids Surf., A, 2001, 189, 265–278 CrossRef CAS.
- I. Pollet, J. Pieters and R. Verschoore, Solar Energy, 2002, 73, 327–335 CrossRef CAS.
- E. Rangel, W. Bento, M. Kayama, W. Schreiner and N. Cruz, Surf. Interface Anal., 2003, 35, 179–183 CrossRef CAS.
- G. Grosu, L. Andrzejewski, G. Veilleux and G. Ross, J. Phys. D: Appl. Phys., 2004, 37, 3350 CrossRef CAS.
- P. Brown, O. Atkinson and J. Badyal, ACS Appl. Mater. Interfaces, 2014, 6, 7504–7511 CAS.
- L. Li, P. Jones and Y.-T. Hsia, Tribol. Lett., 2004, 16, 21–27 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04483a |
| This journal is © The Royal Society of Chemistry 2015 |