Organocatalytic direct difluoromethylation of aldehydes and ketones with TMSCF2H†
Guang-Fen Duab,
Ying Wangb,
Cheng-Zhi Gub,
Bin Dai*b and
Lin He*b
aSchool of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, P. R. China
bSchool of Chemistry and Chemical Engineering, Shihezi University, Xinjiang Uygur Autonomous Region, 832000, P. R. China. E-mail: db_tea@shzu.edu.cn; helin@shzu.edu.cn; Fax: +86-993-2057270
First published on 13th April 2015
Abstract
An organic Lewis base promoted direct difluoromethylation reaction of carbonyl compounds with Me3SiCF2H has been studied. The Schwesinger's superbase can efficiently activate the Si–CF2H bond and initiate the difluoromethylation of aldehydes and ketones under very mild conditions, producing difluoromethyl adducts in 42–99% yields.
Organofluorine compounds have found widespread applications in pharmaceuticals, agrochemicals and medicinal chemistry.1 In particular, the introduction of fluorinated moieties into organic molecules has become a powerful strategy in new drug discovery. As a result, tremendous efforts have been exerted to develop efficient methodologies for the synthesis of fluoroorganics. Among various fluorinated moieties, difluoromethyl group (CF2H) has attracted considerable attention in recent years.2 CF2H is isosteric to hydroxy group (OH) and compared to OH and NH groups, this fluorinated moiety can serve as a lipophilic hydrogen bond donor through hydrogen bonding.3 These unique properties make the CF2H group to be particularly interesting with respect to the design of biologically active molecules. However, compared to the extensively studied trifluoromethylation reactions,4 efficient methods for the introduction of CF2H group are scarce. Similar with the direct nucleophilic trifluoromethylation reactions, the direct difluoromethylation reaction is the most straightforward method for the introduction of CF2H group into organic molecules. However, difluoromethylated organometallic reagents are inefficient for difluoromethylation of carbonyl compounds.5 Difluoromethyl phenylsulfone6 and several difluoromethylsilanes7 can undergo indirect difluoromethylation with carbonyl compounds, but additional reactions are needed to remove the auxiliary groups of the difluoromethylated adducts. Silylated reagent TMSCF2H can be easily prepared from Ruppert–Prakash reagent (TMSCF3).8 In 1995, Fuchikami and Hagiwara documented9 that KF can initiate the direct difluoromethylation reaction of TMSCF2H and carbonyl compounds. However, unlike the analogous Si–CF3 bond, harsh reaction conditions are required for the cleavage of the relatively inert Si–CF2H bond, which restricts the applications of this difluoromethylating reagent for long time. Until recently, Hu and co-workers10 found that CsF or stoichiomeric t-BuOK can activate the Si–CF2H bond efficiently and initiate the difluoromethylation of carbonyl compounds and imines. However, to the best of our knowledge, no other catalysts have been developed for the activation of TMSCF2H and efficient protocol for the direct difluoromethylation is still highly desirable.
In recent years, several organic Lewis bases such as Bu4N+ alkoxides,11 tris(2,4,6-trimethoxyphenyl)phosphine,12 sodium phenoxide–phosphine oxides13 and N-heterocyclic carbenes14 have been utilized successfully for the activation of different silylated reagents. As an important class of superbases, proazaphosphatranes exhibit high reactivity toward the activation of silylated nucleophiles and a variety of transformations have been developed by Verkade and co-workers15 in the past decade. As another important type of superbases, phosphazenes16 have been applied successfully in deprotonative reactions.17 However, phosphazenes catalysed transformations of organosilanes are farless examined. Until recently, Kondo and co-workers18 disclosed that phosphazene bases can be utilized to activate several silylated nucleophiles efficiently. We envisioned that phosphazenes and other organic Lewis bases can be utilized to activate TMSCF2H to initiate the direct difluoromethylation reaction of carbonyl compounds. Herein, we would like to disclose these interesting results.
Our study commenced with the reaction of TMSCF2H and p-chlorobenzaldehyde. To our delight, we found that the reaction proceed smoothly in the presence of 10 mol% t-Bu-P4 (4a), producing difluoromethyl carbinol 3a in 52% yield after 12 h (Table 1, entry 1). Phosphazene bases 4b and 4c can also promote the addition reaction, but in relatively low efficiency (Table 1, entries 2 and 3). To our surprise, N-heterocyclic carbenes 5a–5d, which were proved to be highly efficient catalysts for trifluoromethylation of aldehydes,19 only showed very low efficiency for difluoromethylation reaction of aldehydes (Table 1, entries 4–7). Whereas DBU can't catalyse the addition reaction (Table 1, entry 8). The evaluation of other reaction media indicated the high polar solvent of DMF was the best choice with respect to the reaction yield (Table 1, entries 9–14). Reduction of catalyst loading to 5 mol% led to dramatic decrease of reaction rate (Table 1, entry 15).
| Entry | Conditions | Time (h) | Yieldb (%) |
|---|---|---|---|
| a 1 (1.5 equiv.), 2a (1.0 equiv.).b Isolated yield.c Using 5 mol% 4a. | |||
| 1 | 4a, THF | 12 | 52 |
| 2 | 4b, THF | 12 | 34 |
| 3 | 4c, THF | 12 | 18 |
| 4 | 5a, tBuOK, THF | 72 | 11 |
| 5 | 5b, tBuOK, THF | 72 | 12 |
| 6 | 5c, tBuOK, THF | 72 | <10 |
| 7 | 5d, tBuOK, THF | 72 | <10 |
| 8 | 6, THF | 12 | 0 |
| 9 | 4a, DMF | 0.5 | 91 |
| 10 | 4b, DMF | 2 | 73 |
| 11 | 4c, DMF | 2 | 48 |
| 12 | 4a, PhCH3 | 12 | 23 |
| 13 | 4a, CH2Cl2 | 12 | <10 |
| 14 | 4a, CH3CN | 12 | <10 |
| 15c | 4a, DMF | 12 | 63 |
With the optimized reaction conditions in hand, the substrate scope and the generality of the reaction were subsequently investigated, and the results are summarized in Table 2. Aromatic aldehydes bearing electron-withdrawing, -neutral, and -donating groups can participate in the direct difluoromethylation reaction in excellent yields (Table 2, entries 1–6). Additionally, the substituents on the ortho-, meta-, and para-positions of the aromatic ring showed no obvious effects on the reaction yields (Table 2, entries 7–11). Interestingly, naphthaldehyde and heliotropin were proved to be excellent candidates for the reaction, producing the corresponding difluoromethyl carbinols in excellent yields (Table 2, entries 12–14). A variety of heterocyclic aldehydes, such as furfural, 2-thienal, pyrrole-2-carboxaldehyde, indole-4-carboxaldehyde, isoquinoline-5-carboxaldehyde and 5-coumarancarboxaldehyde can participate in the reaction smoothly, producing the corresponding products in good to high yields (Table 2, entries 15–20). Increasing the catalyst loading to 20 mol%, cinnamaldehyde can also undergo the reaction smoothly to produce 3u in 81% yield (Table 2, entry 21). However, for aliphatic aldehyde 2v, the strong basicity of t-Bu-P4 can cause undesired side reactions, which led to moderate yield of the final fluorinated product (Table 2, entry 22).
| Entry | R | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 10 mol% of 4a, 1.5 equiv. of 1, 0.5 mol L−1 of 2, room temperature for 0.5–3 h.b Isolated yield.c Using 2.0 equiv. TBAF instead of HCl for desilification.d Using 20 mol% of 4a. | ||||
| 1 | 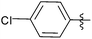 |
0.5 | 3a | 91 |
| 2 |  |
0.5 | 3b | 99 |
| 3 | 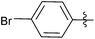 |
0.5 | 3c | 99 |
| 4 |  |
1 | 3d | 99 |
| 5 |  |
1 | 3e | 99 |
| 6 |  |
1 | 3f | 99 |
| 7 |  |
0.5 | 3g | 92 |
| 8 |  |
0.5 | 3h | 92 |
| 9 |  |
0.5 | 3i | 99 |
| 10 |  |
0.5 | 3j | 99 |
| 11 | 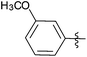 |
0.5 | 3k | 99 |
| 12 |  |
3 | 3l | 95 |
| 13 |  |
3 | 3m | 88 |
| 14 |  |
1 | 3n | 99 |
| 15c |  |
0.5 | 3o | 87 |
| 16c |  |
3 | 3p | 68 |
| 17c |  |
6 | 3q | 66 |
| 18c |  |
6 | 3r | 72 |
| 19c |  |
3 | 3s | 95 |
| 20 |  |
2 | 3t | 99 |
| 21c,d |  |
0.5 | 3u | 81 |
| 22c |  |
1 | 3v | 42 |
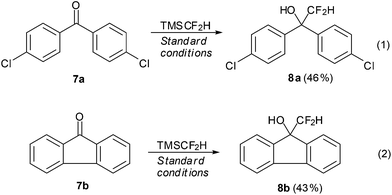
We further applied this protocol to the more challenging ketones. Gratifyingly, under the standard reaction conditions, non-enolizable ketones such as biaryl ketone 7a and fluorenone 7b react with TMSCF2H smoothly to afford the corresponding products in 43% and 46% yields, respectively (Scheme 1).
Based on the pioneering work of Kondo18 and Verkade,15 a plausible mechanism is proposed and illustrated in Scheme 2. t-Bu-P4 attacks the silicon atom of TMSCF2H to form a hexavalent species I, which might trigger the addition to carbonyl compounds and produce oxy anion II. The following migration of TMS from t-Bu-P4 to II led to the formation of III with release of the catalyst. And acidic work up produces the difluoromethyl carbinol.
Conclusions
In conclusion, we have demonstrated an organocatalytic difluoromethylation reaction of aldehydes and ketones. The mild conditions, simple procedure and high yields provide an efficient and novel protocol for the introduction of the significantly important difluoromethyl group into organic molecules, which paves the new way for direct difluoromethylation reactions, although the protocol is not suitable for aliphatic aldehydes and enolizable ketones. Further study of the reaction scope and the applications of this methodology in bioactive compounds synthesis are on-going in our group.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21262027) and the Natural Science Foundation of Shihezi university (no. 2011ZRKETD-04, 2012ZRKXJQ06).Notes and references
- (a) J. Wang, M. Sánchez-Roselló, J. Luis Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (b) P. Kirsch, Modern fluoroorganic chemistry: synthesis, reactivity, applications, Wiley-VCH, Weinheim, 2nd edn, 2013 Search PubMed; (c) I. Ojima, J. Org. Chem., 2013, 78, 6358 CrossRef CAS PubMed; (d) K. Uneyama, J. Fluorine Chem., 2008, 129, 550 CrossRef CAS PubMed; (e) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (f) J. P. Bégué and D. Bonnet-Delpon, Bioorganic and medicinal chemistry of fluorine, Wiley-VCH, Weinheim, 2008 Search PubMed; (g) Fluorine in Medicinal Chemistry and Chemical Biology, ed. I. Ojima, Wiley-Blackwell, Chichester, 2009 Search PubMed; (h) Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, ed. V. Gouverneur and K. Muller, Imperial College Press, London, 2012 Search PubMed.
- For reviews, see: (a) C.-F. Ni, L.-G. Zhu and J.-B. Hu, Acta Chim. Sin., 2015, 73, 90 CrossRef CAS; (b) B. Gao, C. Ni and J. Hu, Chimia, 2014, 68, 414 CrossRef PubMed; (c) P. Xu, S. Guo, L. Wang and P.-P. Tang, Synlett, 2015, 26, 36 CAS; (d) T. Besset, T. Poisson and X. Pannecoucke, Chem.–Eur. J., 2014, 20, 16830 CrossRef CAS PubMed; (e) Y.-L. Liu, J.-S. Yu and J. Zhou, Asian J. Org. Chem., 2013, 2, 194 CrossRef CAS PubMed; (f) J.-B. Hu, W. Zhang and F. Wang, Chem. Commun., 2009, 7465 RSC; (g) C. Ni, M. Hu and J.-B. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed.
- (a) G. K. S. Prakash, M. Mandal, S. Schweizer, N. A. Petasis and G. A. Olah, J. Org. Chem., 2002, 67, 3718 CrossRef CAS PubMed; (b) J. A. Erickson and J. I. McLoughlin, J. Org. Chem., 1995, 60, 1626 CrossRef CAS; (c) Y. Li and J. Hu, Angew. Chem., Int. Ed., 2005, 44, 5882–5886 CrossRef CAS PubMed; (d) G. K. S. Prakash, C. Weber, S. Chacko and G. A. Olah, Org. Lett., 2007, 9, 1863 CrossRef CAS PubMed.
- For selected reviews, see: (a) M. Médebielle and W. R. Dolbier Jr, J. Fluorine Chem., 2008, 129, 930 CrossRef PubMed; (b) J. A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS; (c) J. Nie, H. C. Guo, D. Cahard and J. A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed; (d) G. Valero, X. Companyó and R. Rios, Chem.–Eur. J., 2011, 17, 2018 CrossRef CAS PubMed; (e) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (f) X.-F. Wu, H. Neumann and M. Beller, Chem.–Asian J., 2012, 7, 1744 CrossRef CAS PubMed; (g) T. Besset, C. Schneider and D. Cahard, Angew. Chem., Int. Ed., 2012, 51, 5048 CrossRef CAS PubMed; (h) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (i) J. Xu, X. Liu and Y. Fu, Tetrahedron Lett., 2014, 55, 585 CrossRef CAS PubMed; (j) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 CAS; (k) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed.
- (a) G. A. Hartgraves and D. J. Burton, J. Fluorine Chem., 1988, 39, 425 CrossRef CAS; (b) D. J. Burton, G. A. Hartgraves and J. Hsu, Tetrahedron Lett., 1990, 31, 3699 CrossRef CAS; (c) D. J. Burton and G. A. Hartgraves, J. Fluorine Chem., 2007, 128, 1198 CrossRef CAS PubMed.
- G. K. S. Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921 CrossRef CAS PubMed.
- For an excellent review, see: (a) K. Uneyama, J. Fluorine Chem., 2008, 129, 550 CrossRef CAS PubMedfor selected examples, see: (b) J. Liu, C. Ni, F. Wang and J. Hu, Tetrahedron Lett., 2008, 49, 1605 CrossRef CAS PubMed; (c) L. Zhu, Y. Li, Y. Zhao and J. Hu, Tetrahedron Lett., 2010, 51, 6150 CrossRef CAS PubMed; (d) T. Bootwicha, D. Panichakul, C. Kuhakarn, S. Prabpai, P. Kongsaeree, P. Tuchinda, V. Reutrakul and M. Pohmakotr, J. Org. Chem., 2009, 74, 3798 CrossRef CAS PubMed; (e) Y. Li and J. Hu, J. Fluorine Chem., 2008, 129, 382 CrossRef CAS PubMed; (f) M. Pohmakotr, D. Panichakul, P. Tuchinda and V. Reutrakul, Tetrahedron, 2007, 63, 9429 CrossRef CAS PubMed; (g) S. Mizuta, N. Shibata, S. Ogawa, H. Fujimoto, S. Nakamura and T. Toru, Chem. Commun., 2006, 2575 RSC; (h) G. K. S. Prakash, J. Hu, Y. Wang and G. A. Olah, J. Fluorine Chem., 2005, 126, 527 CrossRef PubMed; (i) C. Ni and J. Hu, Tetrahedron Lett., 2005, 46, 8273 CrossRef CAS PubMed; (j) Y.-Y. Qin, X.-L. Qiu, Y.-Y. Yang, W.-D. Meng and F.-L. Qing, J. Org. Chem., 2005, 70, 9040 CrossRef CAS PubMed.
- A. A. Tyutyunov, V. E. Boyko and S. M. Igoumnov, Fluorine Notes, 2011, 74, 1 CAS.
- T. Hagiwara and T. Fuchikami, Synlett, 1995, 1995, 717 CrossRef PubMed.
- Y. Zhao, W. Huang, J. Zheng and J. Hu, Org. Lett., 2011, 13, 5342 CrossRef CAS PubMed.
- (a) M. Das and D. F. O'Shea, J. Org. Chem., 2014, 79, 5595 CrossRef CAS PubMed; (b) T. Kitazawa, T. Minova and T. Mukaiyama, Chem. Lett., 2006, 35, 1002 CrossRef CAS.
- (a) S. Matsukawa and E. Kitazaki, Tetrahedron Lett., 2008, 49, 2982 CrossRef CAS PubMed; (b) S. Matsukawa and T. Sekin, Synth. Commun., 2009, 39, 1718 CrossRef CAS.
- M. Hatano, E. Takagi and K. Ishihara, Org. Lett., 2007, 9, 4527 CrossRef CAS PubMed.
- For reviews on NHCs mediated transformations of silylated reagents, see: (a) M. J. Fuchter, Chem.–Eur. J., 2010, 16, 12286 CrossRef CAS PubMed; (b) L. He, H. Guo, Y. Wang, G.-F. Du and B. Dai, Tetrahedron Lett., 2015, 56, 972 CrossRef CAS PubMed.
- For reviews, see: (a) J. G. Verkade and P. B. Kisanga, Tetrahedron, 2003, 59, 7819–7858 CrossRef CAS; (b) J. G. Verkade and P. B. Kisanga, Aldrichimica Acta, 2004, 37, 3–14 CAS; (c) S. Urgaonkar and J. G. Verkade, Spec. Chem., 2006, 26, 36–39 CASfor selected examples, see: (d) K. Wadhwa and J. G. Verkade, J. Org. Chem., 2009, 74, 5683 CrossRef CAS PubMed; (e) K. Wadhwa, V. R. Chintareddy and J. G. Verkade, J. Org. Chem., 2009, 74, 6681 CrossRef CAS PubMed; (f) V. R. Chintareddy, K. Wadhwa and J. G. Verkade, J. Org. Chem., 2009, 74, 8118 CrossRef CAS PubMed; (g) K. Wadhwa and J. G. Verkade, J. Org. Chem., 2009, 74, 4368 CrossRef CAS PubMed.
- R. Schwesinger and H. Schlemper, Angew. Chem., Int. Ed. Engl., 1987, 26, 1167–1169 CrossRef PubMed.
- For an excellent review, see: (a) T. Ishikawa, Superbases for Organic Synthesis, John Wiley & Sons Ltd, Chichester, 2009 Search PubMedfor selected examples, see: (b) T. Imahori and Y. Kondo, J. Am. Chem. Soc., 2003, 125, 8082 CrossRef CAS PubMed; (c) K. Kobayashi, M. Ueno and Y. Kondo, Chem. Commun., 2006, 3128 RSC; (d) M. Ueno, M. Yonemoto, M. Hashimoto, A. E. H. Wheatley, H. Naka and Y. Kondo, Chem. Commun., 2007, 2264 RSC; (e) K. Kobayashi, M. Ueno, H. Naka and Y. Kondo, Chem. Commun., 2008, 3780 RSC; (f) K. Kobayashi, M. Ueno, H. Naka and Y. Kondo, Chem.–Eur. J., 2009, 15, 9805 CrossRef CAS PubMed; (g) Y. Araki, K. Kobayashi, M. Yonemoto and Y. Kondo, Org. Biomol. Chem., 2011, 9, 78 RSC; (h) H. Kawai, Z. Yuan, E. Tokunaga and N. Shibata, Org. Biomol. Chem., 2013, 11, 1446–1450 RSC.
- (a) M. Ueno, C. Hori, K. Suzawa, M. Ebisawa and Y. Kondo, Eur. J. Org. Chem., 2005, 2005, 1965–1968 CrossRef PubMed; (b) K. Suzawa, M. Ueno, A. E. H. Wheatley and Y. Kondo, Chem. Commun., 2006, 4850–4852 RSC; (c) H. Naka, D. Koseki and Y. Kondo, Adv. Synth. Catal., 2008, 350, 1901 CrossRef CAS PubMed.
- J. J. Song, Z. Tan, J. T. Reeves, F. Gallou, N. K. Yee and C. H. Senanayake, Org. Lett., 2005, 7, 2193 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04472c |
| This journal is © The Royal Society of Chemistry 2015 |