A simple two-step silane-based (bio-) receptor molecule immobilization without additional binding site passivation†
Abstract
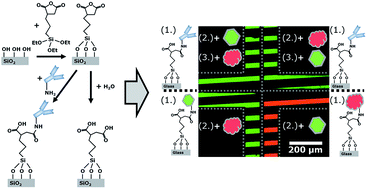
We present a simple method for immobilizing (bio-) receptor molecules on glass surfaces using the silane 3-(triethoxysilyl)propylsuccinic anhydride (TESPSA). Its succinic anhydride functionality enables the covalent binding of amino-terminated molecules in a ring opening reaction under formation of an amide bond. We demonstrate proof-of-concept using fluorescence microscopy that antibodies immobilized with the developed method maintain their specificity for their fluorescently labelled analytes, and that no additional binding site passivation is required. Therefore, the presented method significantly facilitates the functionalization of surfaces for a wide range of biosensor systems.

 Please wait while we load your content...
Please wait while we load your content...