Size dependence and UV irradiation tuning of the surface potential in single conical ZnO nanowires†
Zengze Wanga,
Yousong Gua,
Junjie Qia,
Shengnan Lua,
Peifeng Lia,
Pei Lina and
Yue Zhang*ab
aState Key Laboratory for Advanced Metals and Materials, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, People’s Republic of China
bKey Laboratory of New Energy Materials and Technologies, University of Science and Technology Beijing, Beijing 100083, People’s Republic of China. E-mail: yuezhang@ustb.edu.cn
First published on 14th April 2015
Abstract
Investigating and tailoring surface potential changes of a system at the interfaces is of significance in the fundamental understanding and application of semiconductor devices. Thus the surface potential of zinc oxide (ZnO) nanowires is a vital factor to tune the performance of devices. In this paper, Kelvin probe force microscopy (KPFM) is used to measure the surface potential of single conical ZnO nanowires with different diameters. A size dependence of the surface potential in single conical ZnO nanowires is experimentally revealed. As the diameter decreases, the surface potential of the ZnO nanowires is found to decrease linearly under 400 nm. At large diameters (≥400 nm), the surface potential remains almost constant. The contact potential difference of the ZnO–PtIr tip increases to saturation after 40 min UV illumination and remains stable. An energy band theory is introduced to explore the surface potential change of ZnO nanowires under UV illumination. This study provides an understanding of the surface electrical properties of semiconductors at the nanoscale, which is valuable for optimizing functional nanodevices based on semiconductor nanowires.
“The interface is the device” was coined by Nobel laureate Herbert Kroemer.1 While the work function of semiconductor nano-materials is a vital factor to determine the interface of nano-devices, in the classic semiconductor theory, when a semiconductor comes into contact with a metal, a Schottky barrier is formed at the metal–semiconductor interface. This barrier is responsible for controlling the current conduction as well as capacitance behavior.2 The barrier height is produced from the difference between the metal work function and the electron affinity of the semiconductor. In practice, however, simple expressions for the barrier heights as above-mentioned have never been realized in any laboratories. The main deviations of experimental barrier heights from the ideal conditions are unavoidable due to the interface layer and the presence of interface states. We assume that the surface potential of a semiconductor is the work function combined with the surface states of the interface layer. Thus it is essential to study and experimentally quantify the surface potential relationship of semiconductors for fabrication of nanodevices. Thus several scientists have investigated the modulation of surface states of ZnO nanowires.3,4 Martin et al. investigated the surface states of ZnO using various methods.5,6
The understanding of nanomaterial surface potential properties has brought about much progress in nanodevices and their integration.7 Kelvin probe force microscopy (KPFM) was selected to measure the surface potential distribution of single ZnO nanowires. KPFM is a powerful and facilitative technique for studying nanoscale surface properties.8 KPFM has found some applications in various fields, such as dopant profiling in semiconductors,9 the study of operating light-emitting devices,10 thin film solar cells,11 corrosion science,12 and so on. Many variants of KPFM techniques have evolved and are discussed in various book chapters or review articles.13,14 Therefore this technique is conveniently used to investigate various nanomaterials. Yang et al. demonstrated the electronic properties of As-doped homojunction ZnO nanorods using KPFM.9 Wang et al. investigated the potential distribution along a bent ZnO microwire (MW) body under optimal external bias using KPFM.15 Soudi et al. demonstrated diameter dependent surface photovoltage and surface state density in single ZnO nanowires.16 Although size effects of several characteristics of ZnO nanowires, such as electric conductivity,17 mechanism properties,18,19 dielectric constant,20 have been reported, few studies have reported the size effect of the surface potential of identical nanowires with increasing diameters so far. Moreover, the surface potential of ZnO nanowires has a significant role in tuning the performance of nanodevices. When the surface potential of a semiconductor material increases, the Schottky barrier formed by the metal and semiconductor could be magnified. In contrast, the contact of the metal and semiconductor would change to ohmic type, while the surface potential of ZnO nanowires reduces to the value of the metal work function. Therefore it is more important to understand how the surface potential of single nanomaterials changes with decreasing diameter before it can be used to fabricate nanodevices.
In this paper, we conducted scanning probe microscopy (SPM) experiments, using KPFM in particular, to investigate the surface potentials of single conical ZnO nanowires with changing diameters. Our results show that the surface potential of ZnO nanowires increases with an increase of diameter at the small scale (<400 nm) and is kept stable at the large scale (>400 nm). UV irradiation was introduced to tune the surface potential of the ZnO nanowires.
The conical ZnO nanowires were achieved from ZnO nano-tetrapods. A simple chemical vapour deposition (CVD) process was carried out to synthesize the ZnO nano-tetrapods.21 In brief, ZnO nano-tetrapods were synthesized on a silicon substrate by a simple chemical vapour deposition method. A mixture of zinc (99.9%) and ZnAc powders with a molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used as the evaporation source in a quartz boat. A Si wafer cleaned by ultrasonication in acetone, ethanol and DI water was used as the substrate to collect the products. Then the quartz boat was placed at the centre of a tube furnace. The flow rate of Ar/O2, growth time and temperature were maintained at 297/3 SCCM (SCCM denotes standard cubic centimetre per minute at STP), 15 min and 650 °C. After the reaction, a white product, obtained on the substrate, is the ZnO nano-tetrapods. Then the ZnO nano-tetrapods were dispersed in ethanol and broken down by ultrasonication at 300 W for 30 min. Every single leg of the acquired ZnO nano-tetrapods was a conical shape. The conical ZnO nanowires were transferred onto a silicon substrate covered with a Au film. The Au film was deposited using a DC sputtering method. To fix the nanowires, a thin rectangular Pt layer was deposited on the bigger terminal of the wires using focused ion beam (FIB, FEI Quanta 3D FEG) induced deposition. Photoluminescence (PL) measurements were carried out on a Raman spectrometer (Jobin-Yvon, HR800) with a 325 nm He–Cd laser source at room temperature.
1 was used as the evaporation source in a quartz boat. A Si wafer cleaned by ultrasonication in acetone, ethanol and DI water was used as the substrate to collect the products. Then the quartz boat was placed at the centre of a tube furnace. The flow rate of Ar/O2, growth time and temperature were maintained at 297/3 SCCM (SCCM denotes standard cubic centimetre per minute at STP), 15 min and 650 °C. After the reaction, a white product, obtained on the substrate, is the ZnO nano-tetrapods. Then the ZnO nano-tetrapods were dispersed in ethanol and broken down by ultrasonication at 300 W for 30 min. Every single leg of the acquired ZnO nano-tetrapods was a conical shape. The conical ZnO nanowires were transferred onto a silicon substrate covered with a Au film. The Au film was deposited using a DC sputtering method. To fix the nanowires, a thin rectangular Pt layer was deposited on the bigger terminal of the wires using focused ion beam (FIB, FEI Quanta 3D FEG) induced deposition. Photoluminescence (PL) measurements were carried out on a Raman spectrometer (Jobin-Yvon, HR800) with a 325 nm He–Cd laser source at room temperature.
Conductive atomic force microscope (C-AFM) PtIr tips (SCM-PIC) with a spring constant of about 0.2 N m−1 were used in the KPFM experiments with a lift model (double-pass imaging operation). In the first pass, the topography profile was obtained using the standard tapping mode. In the second line scan, the AFM tip was lifted 50 nm above the topographical baseline. KPFM was applied with a dc-voltage to compensate for the contact potential difference (CPD) between the AFM tip and the sample.22 KPFM records this difference as a function of tip position, which is taken on a commercially available AFM (Nanoscope IIID, Multimode).
Fig. 1a shows the morphology of a conical ZnO nanowire which was fixed by a FIB. It is clear from Fig. 1a that the diameters of the ZnO nanowire changes significantly especially at the terminals of the nanowire. Fig. 1b displays the PL spectrum of the ZnO nanowires at room temperature. The first peak, centered at 380 nm, indicates the near-band-edge UV emission in the UV region and free-exciton peak of ZnO.23 The strong visible emission peak (520 nm) suggests that a large number of oxygen vacancies exist in the ZnO nanowires. Fig. 1c shows the schematic image of the measurement system setup.
 | ||
| Fig. 1 (a) SEM image of a ZnO conical nanowire fixed by a FIB. (b) PL spectrum of the ZnO nanowires. (c) Schematic diagram of the measurement system setup. | ||
KPFM allows the imaging of surface electronic properties, namely the CPD, with nanometer scale resolution.13 The CPD between two materials depends on a variety of parameters such as the work function, adsorption layers, oxide layers, dopant concentration in semiconductors, or temperature changes of the sample.24,25 The CPD between the AFM tip and a sample, is defined as
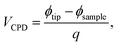 | (1) |
In addition to the measurement of the compensation dc-voltage (Vdc) between the tip and sample, an ac-voltage Vac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(ωact) at the frequency ωac is applied. The resulting oscillating electrostatic force induces an oscillation of the cantilever at the frequency ωac. Considering the tip–sample system as a capacitor, the electrostatic force can now be expressed as:
sin(ωact) at the frequency ωac is applied. The resulting oscillating electrostatic force induces an oscillation of the cantilever at the frequency ωac. Considering the tip–sample system as a capacitor, the electrostatic force can now be expressed as:
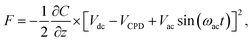 | (2) |
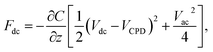 | (3) |
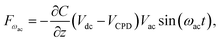 | (4) |
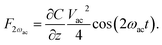 | (5) |
Fig. 2a shows a typical AFM image of a single conical ZnO nanowire with the diameter ranging from 50 to 500 nm on the Si substrate coated with Au film and its 3-dimensional (3D) AFM image. The corresponding surface potential image (Fig. 2b) shows that the CPD of the tip and the ZnO nanowire declines with decreasing diameter.
The cross sections of the height signals are displayed in Fig. 2c and have been taken at the ZnO nanowire positions indicated by the red dashed line in Fig. 2a. It is clearly seen that the height decreases along the axial direction, especially at the terminal of the nanowire. The three cross sections of the height signals indicated by the white dashed line in Fig. 2a are shown in Fig. 2d. We can see from the figure that the height increases from 258 nm to 350 nm as the position changes.
The cross sections of CPD signals along the axial direction are shown in Fig. 2e as indicated by the red dashed line in Fig. 2b. The CPD of the tip–ZnO nanowire decreases along the axial direction. According to the data of Fig. 2c and e, the relationship of the ZnO nanowire diameters vs. CPD is plotted in Fig. 2f. At small diameters (<400 nm), the increase in CPD was almost linear to the increasing diameter. With the increase in diameter, the growing tendency of the CPD was greatly reduced. At large diameters (>400 nm), the CPD remains stable. According to formula (1), the ϕsample (the surface potential of the ZnO nanowires) declines with increasing CPD, and the conductive band of ZnO in the band diagram is raised.
As is well known, the surface potential of a sample depends on the surface dipole (Δφs), the surface band bending (eVs), the electron affinity (χ), and the carrier concentration.28 The termination of the periodic structure of a semiconductor at its free surface may form surface-localized electronic states within the semiconductor bandgap and/or a double layer of charge, known as a surface dipole (Δφs).29 Therefore, the surface potential can be written as30
| VSP = (EC − EF) + χ + eVs − Δφs | (6) |
The electron density could be calculated according to semiconductor theory (see ESI†). The surface potential decreases with increasing electron density. We assume that surface state change is the crucial factor for tuning surface potential. The trapping at surface states drastically reduces the surface potential properties.14 For an n-type semiconductor nanowire, acceptor-like surface states, when occupied by electrons, carry negative charges and induce upward surface band bending as well as a surface depletion of electrons in the semiconductor.16 ZnO nanowires with small diameters have much more trapped charge density, due to high surface-to-volume ratios. In general, the surface state density increases with decreasing diameter.16 Therefore the surface potential of a ZnO nanowire decreases with declining diameter, when the diameter of a ZnO nanowire is smaller than 400 nm. When the diameter is larger than 400 nm, the surface state density reaches a minimum. Thus the surface potential remains stable.
UV illumination at 200 μW cm−2 and a wavelength centered at 375 nm was introduced to tune the surface state of the ZnO nanowires. Under the UV illumination, we measured the surface potential of the samples under ambient conditions. It is noted that the oxygen-rich environment of ZnO influences the surface potential imaging of charge-injected regions, which has also been reported in the semiconductors by others.28
The corresponding surface potential images of a single ZnO nanowire under UV illumination from 0 min to 140 min are shown in Fig. 3. It can be seen from the figures that the surface potential of the ZnO nanowires increases with the illumination time. To accurately know the tendency of the surface potential, the cross sections of the CPD are displayed in Fig. 4a and the ZnO nanowire position is indicated by the red dashed line in Fig. 3a. It can be seen from Fig. 4a, that the surface potential of the ZnO nanowire increases rapidly in the first 40 min and remains stable in the subsequent time. The CPD of the tip and ZnO nanowires with different diameters are shown in Fig. 4b. The surface potential of a ZnO nanowire with a small diameter increases slowly in the range of 140 min. By contrast, the surface potential of a ZnO nanowire with a large diameter increases sharply in the first 40 min and then remains stable.
 | ||
| Fig. 3 (a–h) The corresponding surface potential images of a ZnO nanowire under UV illumination from 0 min to 140 min. | ||
 | ||
| Fig. 4 (a) The cross sections of the CPD under UV illumination from 0 min to 140 min. (b) The cross sections of the CPD (ZnO–tip) at different diameters ranging from 28 nm to 433 nm. | ||
It is clear that the CPD of the ZnO nanowire–tip increases in the first 40 min and then reaches saturation. In the dark, the surface potential of a ZnO nanowire, about 433 nm in diameter, was about 98.1 mV higher than that of the PtIr tip, mainly due to the work function difference between them. The change in the surface potential of the ZnO under UV illumination depends on carrier density, the effective Fermi level, and surface states. After UV illumination, the CPD of the ZnO nanowire and PtIr tip increased to 129.3 mV, indicative of a change in the effective Fermi level of ZnO. The majority of the photogenerated electrons are coming from the valence band edge of the ZnO rather than possible effect states or hot carriers.31 The electron density of a ZnO nanowire with 300 nm diameter increased from 2.45 × 1017 to 2.68 × 1017 (see ESI†). At the same time the surface potential of the ZnO nanowire decreased from 5.58 eV to 5.49 eV. Thus the surface potential of the ZnO nanowire decreases as the carrier density increases. Fig. 5 shows a schematic of the surface potential change mechanism in the presence of a high density of hole-trap states, hydroxyl and adsorbed oxygen ions at the nanowire surface.
The surface photovoltage (eVs) is defined as the change in the surface potential due to surface band bending. There acceptor-like surface states occupied by electrons induce the upward surface band bending. Under UV illumination, negatively charged surface states capture the photogenerated holes and become neutral, and this reduces the surface band bending. Upon illumination, electron–hole pairs are photogenerated and holes are readily trapped at the surface, leaving behind unpaired electrons, which decrease the surface potential of the ZnO nanowire.32 The occupied surface states of ZnO nanowires are depopulated upon UV illumination with an appropriate light energy.33 Schematics of the ZnO NW energy band diagrams in the dark and under UV illumination are shown in Fig. 5. In the dark, oxygen molecules are adsorbed on the ZnO NW surface and capture the free electrons present in the semiconductor. When the ZnO NW is dipped in ethanol, this material reacts with the adsorbed oxygen molecules and the surface potential is reduced (R–CH2–OH + O– → R–CHO or R–COOH + H2O + e).4,34 Upon UV illumination, electron–hole pairs were generated, holes migrate to the surface and the reactive hydroxyl was reduced to ethanol. Furthermore, the negatively charged adsorbed oxygen ions are discharged, and oxygen is photo-desorbed from the surface. The work function of the ZnO decreased as the electron density increased and the oxygen ions desorbed, as depicted in Fig. 5. When the diameter of the ZnO nanowire is 28 nm, the CPD increases from −79 mV to −26 mV. As the trapped oxygen reached a minimum, the surface potential of the ZnO under UV illumination was saturated.
Conclusions
In summary, a size dependence of the surface potential in single conical ZnO nanowires is experimentally revealed. At small diameters (<400 nm), the surface potential of the ZnO increases linearly. The surface potential of the ZnO reaches a minimum value when the diameters were increased to 400 nm. The CPD of the ZnO NW–PtIr tip increases during the first 40 minutes and then remains stable under UV irradiation. Our investigations could be helpful in the design and improvement of photo-electrochemical and electromechanical devices used for energy conversion applications.Acknowledgements
This work was supported by the National Major Research Program of China (2013CB932600), the Program of International S&T Cooperation (2012DFA50990), NSFC (51232001, 51172022), the Research Fund of Co-construction Program from Beijing Municipal Commission of Education, and the Fundamental Research Funds for the Central Universities, Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- Nat. Mater., 2012, 11, 91 Search PubMed.
- S. M. Sze and K. K. Ng, Physics of Semiconductor Devices, Wiley, New York, 2nd edn, 2007 Search PubMed.
- T. G. G. Maffeis, M. W. Penny, A. Castaing, O. J. Guy and S. P. Wilks, Surf. Sci., 2012, 606, 99–103 CrossRef CAS PubMed.
- A. M. Lord, T. G. Maffeis, M. W. Allen, D. Morgan, P. R. Davies, D. R. Jones, J. E. Evans, N. A. Smith and S. P. Wilks, Appl. Surf. Sci., 2014, 320, 664–669 CrossRef CAS PubMed.
- M. W. Allen, C. H. Swartz, T. H. Myers, T. D. Veal, C. F. McConville and S. M. Durbin, Phys. Rev. B, 2010, 81, 075211 CrossRef.
- R. Heinhold, G. T. Williams, S. P. Cooil, D. A. Evans and M. W. Allen, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 235315 CrossRef.
- G. H. Buh, H. J. Chung, J. H. Yi, I. T. Yoon and Y. Kuk, J. Appl. Phys., 2001, 90, 443 CrossRef CAS PubMed.
- Y. Zhang, X. Yan, Y. Yang, Y. Huang, Q. Liao and J. Qi, Adv. Mater., 2012, 24, 4647–4655 CrossRef CAS PubMed.
- C. V. Ben, H. D. Cho, T. W. Kang and W. Yang, Surf. Interface Anal., 2012, 44, 755–758 CrossRef CAS PubMed.
- R. Shikler, T. Meoded, N. Fried, B. Mishori and Y. Rosenwaks, J. Appl. Phys., 1999, 86, 107 CrossRef CAS PubMed.
- H. Hoppe, T. Glatzel, M. Niggemann, A. Hinsch, M. Lux-Steiner and N. S. Sariciftci, Nano Lett., 2005, 5, 269–274 CrossRef CAS PubMed.
- M. Rohwerder and F. Turcu, Electrochim. Acta, 2007, 53, 290–299 CrossRef CAS PubMed.
- S. Sadewasser and T. Glatzel, Kelvin Probe Force Microscopy, Berlin, 2012 Search PubMed.
- W. Melitz, J. Shen, A. C. Kummel and S. Lee, Surf. Sci. Rep., 2011, 66, 1–27 CrossRef CAS PubMed.
- D. Geng, A. Pook and X. Wang, Nano Energy, 2013, 2, 1225–1231 CrossRef CAS PubMed.
- A. Soudi, C. H. Hsu and Y. Gu, Nano Lett., 2012, 12, 5111–5116 CrossRef CAS PubMed.
- Y. Yang, J. Qi, W. Guo, J. Zhao, X. Wang and Y. Zhang, Appl. Phys. Lett., 2010, 96, 152101 CrossRef PubMed.
- C. Q. Chen, Y. Shi, Y. S. Zhang, J. Zhu and Y. J. Yan, Phys. Rev. Lett., 2006, 96, 075505 CrossRef CAS.
- P. Li, Q. Liao, S. Yang, X. Bai, Y. Huang, X. Yan, Z. Zhang, S. Liu, P. Lin, Z. Kang and Y. Zhang, Nano Lett., 2014, 14, 480–485 CrossRef CAS PubMed.
- Y. Yang, W. Guo, X. Wang, Z. Wang, J. Qi and Y. Zhang, Nano Lett., 2012, 12, 1919–1922 CrossRef CAS PubMed.
- H. Li, Y. Huang, Y. Zhang, J. Qi, X. Yan, Q. Zhang and J. Wang, Cryst. Growth Des., 2009, 9, 1863–1868 CAS.
- J. M. R. Weaver and D. W. Abraham, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 1991, 9, 1559–1561 CrossRef CAS.
- Y. Yang, J. Qi, W. Guo, Y. Gu, Y. Huang and Y. Zhang, Phys. Chem. Chem. Phys., 2010, 12, 12415–12419 RSC.
- P. P. Craig, Rev. Sci. Instrum., 1970, 41, 258 CrossRef PubMed.
- M. Nonnenmacher, M. P. O’Boyle and H. K. Wickramasinghe, Appl. Phys. Lett., 1991, 58, 2921 CrossRef PubMed.
- Y. Rosenwaks, R. Shikler, T. Glatzel and S. Sadewasser, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 085320 CrossRef.
- B.-Y. Tsui, C.-M. Hsieh, P.-C. Su, S.-D. Tzeng and S. Gwo, Jpn. J. Appl. Phys., 2008, 47, 4448–4453 CrossRef CAS.
- H. Kim, S. Hong and D.-W. Kim, Appl. Phys. Lett., 2012, 100, 022901 CrossRef PubMed.
- L. Kronik and Y. Shapira, Surf. Interface Anal., 2001, 31, 954–965 CrossRef CAS PubMed.
- A. Rothschild, A. Levakov, Y. Shapira, N. Ashkenasy and Y. Komem, Surf. Sci., 2003, 532–535, 456–460 CrossRef CAS.
- H. Yoo, C. Bae, Y. Yang, S. Lee, M. Kim, H. Kim, Y. Kim and H. Shin, Nano Lett., 2014, 14(8), 4413–4417 CrossRef CAS PubMed.
- C. Soci, A. Zhang, B. Xiang, S. A. Dayeh, D. P. Aplin, J. Park, X. Y. Bao, Y. H. Lo and D. Wang, Nano Lett., 2007, 7, 1003–1009 CrossRef CAS PubMed.
- A. Henning, G. Gunzburger, R. Johr, Y. Rosenwaks, B. Bozic-Weber, C. E. Housecroft, E. C. Constable, E. Meyer and T. Glatzel, Beilstein J. Nanotechnol., 2013, 4, 418–428 CrossRef PubMed.
- Q. Zhang, J. Qi, Y. Huang, X. Li and Y. Zhang, Mater. Chem. Phys., 2011, 131, 258–261 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04467g |
| This journal is © The Royal Society of Chemistry 2015 |


