Electrochemical synthesis of pillared layer mixed ligand metal–organic framework: DMOF-1–Zn
Sadegh
Khazalpour
a,
Vahid
Safarifard
b,
Ali
Morsali
*b and
Davood
Nematollahi
*a
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan, 65178-38683, Iran. E-mail: nemat@basu.ac.ir
bDepartment of Chemistry, Faculty of Sciences, Tarbiat Modares University, Tehran, Iran. E-mail: morsali_a@modares.ac.ir
First published on 15th April 2015
Abstract
In this paper, a pillared layer mixed ligand metal–organic framework (MOF), namely, DMOF-1–Zn was synthesized via anodic dissolution in an electrochemical cell. The influence of different reaction parameters such as solvent, additive and current density on the textural properties of the MOF obtained were investigated. The characterization of the samples involved powder X-ray diffraction, infrared Fourier transform spectroscopy, and field emission scanning electron microscopy. Moreover, the electrochemical route was successfully combined with a coordination modulation approach to prepare specific morphology at the nano-scale. The main advantages of electrosynthesis are the shorter synthesis time, the milder conditions, the facile synthesis of MOF nanostructures and the morphology tuning.
The field of metal–organic frameworks (MOFs) has been growing tremendously over the last two decades.1,2 This fascinating class of crystalline hybrid materials, which are formed by association of metal centers and organic ligands, offers a unique chemical versatility combined with a designable framework and an unprecedentedly large and permanent inner porosity.3–6 The importance of choosing a proper synthetic route in designing MOF materials with specific sizes, morphologies, and nanostructures has been a driving force for development of new methodologies for several years.7 Recent advances in nanostructured MOFs have been led by development of new synthetic strategies that provide control over size, morphology, and nanostructure.8,9
The utilization of electrochemistry for the synthesis of MOFs offers a facile, environmentally friendly, and versatile synthetic tool for these nanostructured materials that are often unavailable by conventional routes.10,11 The facile synthesis of MOFs is very important for their viable applications in industry.12 The electrochemical synthesis of MOFs was first reported in 2005 by Mueller and coworkers.13 One of the main advantages of the electrochemical method for preparation of MOFs is the possibility to run a continuous process and hence the possibility to obtain a higher solids content compared to normal batch reactions that is an important issue for industrial process.14 Electrochemical synthesis allows preparation under mild conditions and reduced reaction time. Whereas conventional solvothermal synthesis takes days, electrochemical routes can prepare the framework within minutes or hours.15 The principle relies on supplying the metal ion by anodic dissolution to the solution mixture that contains the organic linker and an electrolyte.16,17
Electrochemical synthesis allows more control to be exercised over the reactant concentration in the synthesis over the course of time since it is performed without building pressure and the metal can be added to the solution at different rates by controlling the anodic oxidation.18 In addition, it should be possible to carefully control the oxidation state of the metal simply by adjusting the voltage or current provided to the electrode.
Electrochemical synthesis of MOFs can be performed in batch mode or in continuous flow operation. In the case studies presented in this paper, the experiments were performed in batch mode. The energy required to oxidize the anode can be provided in potentiostatic or galvanostatic modes. In the first case the speed at which the metal ions are dissolved depends on the applied voltage. In galvanostatic mode, the current through the cell is fixed. In this case, the anodic dissolution rate of metal increases, when the applied current density increases. In both methods, because of the low conductivity of the electrolysis medium, supporting electrolytes need to be used.
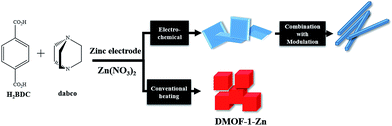
To date the electrochemical formation of microcrystalline powders and films of some famous MOFs such as HKUST-1 (ref. 11 and 18–20) and ZIFs21 were reported using different types of anode materials (Zn, Cu, Mg, Co) and linkers (1,3,5-H3BTC, 1,2,3-H3BTC and H2BDC). In this work, we present the electrochemical synthesis of DMOF-1 based on zinc metal and 1,4-benzenedicarboxylic acid as a O-donor and 1,4-diazabicyclo[2.2.2]octane as a N-donor linkers to form the pillared-layered mixed-ligand metal–organic framework using potentiometric method and lithium perchlorate as a electrolyte Scheme 1.
[Zn2(1,4-BDC)2(DABCO)] was first reported in 2004 named DMOF-1–Zn (Fig. 1).22 Since then, it has become one of the most studied MOFs.23–26 It is frequently prepared under hydrothermal conditions with a zinc salt (usually nitrate), 1,4-benzenedicarboxylic acid (H2BDC) and 1,4-diazabicyclo[2.2.2]octane (dabco). The precursors are dissolved and mixed in dimethylformamide (DMF), and finally synthesized at 110 °C for 2 days. The gas sorption and separation capacity of DMOF-1–Zn have been studied by several research groups.27–33
In order to limit the influence of linker concentration during electrochemical synthesis, a saturated solution of the linkers and an electrolyte is put in contact with two zinc electrodes. By applying a given current, the Zn in the electrode is oxidized to Zn2+ in the solution. Standard synthesis solution of 0.25 mmol (41.5 mg) H2BDC and 0.125 mmol (14 mg) of dabco and 3 mmol (320 mg) of LiClO4 dissolved in 30 ml DMF is placed in contact with two zinc electrodes separated by 2.5 cm. Then, current density of 0.4 mA cm−2 is passed through the system at room temperature. A few minutes after the start of the electrolysis, a white solid started to precipitate. After 6 h, the solid precipitate was filtered off and washed with DMF. Afterwards the sample was dried under vacuum at 60 °C for 12 h and then kept under nitrogen atmosphere. On average ∼52.5 mg of dried DMOF-1–Zn is obtained from the synthesis (73% yield). Fig. 2 shows the simulated XRD pattern from single crystal X-ray data of DMOF-1–Zn (Fig. 2a) in comparison with the XRD pattern of the single crystals DMOF-1–Zn as synthesized (Fig. 2b) and a typical sample of DMOF-1–Zn prepared by the electrochemical process (Fig. 2c). Acceptable matches, with slight differences in 2θ, were observed between the simulated and experimental powder X-ray diffraction patterns.29 The FT-IR spectra of the nano-structure produced by electrochemical method and the bulk material produced by solvothermal route are indistinguishable (Fig. 3a and b).
The current density can be used to control the amount of Zn2+ present in the solution. In order to investigate the role of the current density on the size and morphology of nano-structured DMOF-1–Zn, the above processes were done with four different current densities. Fig. 4 shows the field emission scanning electron microscopy (FE-SEM) of DMOF-1–Zn prepared in different current densities of 0.4, 0.8, 1.6 and 4.8 mA cm−2. Comparison between the samples with different current densities shows that the nano-rod DMOF-1–Zn obtains using lower current density of 0.4 mA cm−2 (Fig. 4a and b), while increasing the current density up to 0.8 mA cm−2 prepare the fine nano-plate morphology (Fig. 4c and d). Moreover, when the current density goes up to 1.6 or 4.8 mA cm−2 the micro-particles were obtained (Fig. 4e–h). The results indicate that the size and morphology of the DMOF-1–Zn is strongly current dependent. The FT-IR spectrum and PXRD patterns of typical samples of DMOF-1–Zn prepared by the electrochemical process at current densities of 0.8, 1.6 and 4.8 mA cm−2 are also same with the crystalline sample. Moreover, to investigation the effect of solvent, the electrochemical process was done in the mixture of DMF and water at current densities of 0.8 mA cm−2. The DMF–water ratio not only affects linker solubility, but also solution conductivity and the deprotonation of the BDC acid. The lower the ratio is, the higher the conductivity will be, and the higher the ratio is, the higher the solubility and the deprotonation will be. Fig. 5a and b show the influence of the water content on the morphology of the products using 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for DMF–water. It seems that the electrosynthesis of the MOF in the lake of water can be obtained more fine plates (see Fig. 4c and d).
1 ratio for DMF–water. It seems that the electrosynthesis of the MOF in the lake of water can be obtained more fine plates (see Fig. 4c and d).
 | ||
| Fig. 4 Electrochemical prepared samples of DMOF-1–Zn obtained in different current densities of (a and b) 0.4, (c and d) 0.8 (e and f) 1.6 and (g and h) 4.8 mA cm−2; in two different scale bars. | ||
 | ||
Fig. 5 Nanostructures of DMOF-1–Zn prepared via (a and b) mixed solvents of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (20 DMF (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80), and (c and d) electrochemical-coordination modulation combined method; in two different scale bars. 80), and (c and d) electrochemical-coordination modulation combined method; in two different scale bars. | ||
The mixed-ligand 3D framework [Zn2(1,4-BDC)2(dabco)] (DMOF-1–Zn) has a tetragonal crystal system, in which the dicarboxylate layer ligands (1,4-BDC) link to the dizinc clusters to form two-dimensional square lattices, which are connected by amine pillar ligands (dabco) at the lattice points.23 The anisotropic framework feature of DMOF-1–Zn, dominated by two coordination modes (1,4-BDC-zinc and dabco-zinc), allows the selective modulation of one of the coordination modes (1,4-BDC-zinc) by adding a monocarboxylic acid as the modulator.34–36 Very recently, the deposition of highly crystalline UiO-66 film on the anode is reported using acetic acid as a modulator.37 To prepare DMOF-1–Zn nanostructures, we combined the coordination modulation approach with electrochemical route.38,39 Nanocrystals of DMOF-1–Zn were synthesized by adding a solution of H2BDC (0.25 mmol) and dabco (0.125 mmol) in DMF to a solution of Li(ClO4) (3 mmol) in 30 ml DMF in the presence of 2 ml acetic acid as a capping reagent (modulator) via current of 0.8 mA cm−2 passed through the system at room temperature for 3 h. As shown in Fig. 2d and 3c the both of PXRD pattern and FT-IR spectra of the sample prepared by the electrochemical-coordination modulation combined method are in the good agreement with the pattern and spectra of as-synthesized one, respectively. As we expected, the uniform nano rod-like morphology for DMOF-1–Zn obtained using the combined method that shows the successful combination between these two routes (Fig. 5c and d).40
To examine the thermal stability of the nanostructures and the single crystals of DMOF-1–Zn, thermal gravimetric analyses (TGA) were carried out between 25 and 600 °C under nitrogen flow (Fig. 6). The TGA data indicate that DMOF-1–Zn loses its guest molecules in the temperature range of 120–200 °C (calcd weight loss for 4DMF + 1/2H2O: 34.5%; found: 34.19%), and the resulting porous framework starts to decompose after 300 °C. The nano-sized compound is less stable and starts to decompose at 100 °C. Detectable decomposition of the nano-particles of DMOF-1–Zn thus starts about 20 degree earlier than that of its bulk counterparts, probably due to the much higher surface to volume ratio of the nano-particles, as more heat is needed to annihilate the lattices of the bulk materials.
 | ||
| Fig. 6 Thermal behavior of compound DMOF-1–Zn as solvothermal (red) and electrochemical (green) synthesized samples. | ||
In conclusion, the mixed-ligand 3D framework DMOF-1–Zn has been synthesized via electrochemical approach. The selective change of the current density allowed us to control the resulting crystal morphology. Also it is shown that electrochemical approach can be combined with coordination modulation method to obtain the nano rod-like morphology. The results reveal that electrochemical approach can be used for not only the synthesis of uniform nanocrystals but also the control of the size and morphology of the materials. The approach is expected to synthesize various nanosized mixed-ligands MOFs.
Acknowledgements
Support of this investigation by Tarbiat Modares University and Bu-Ali Sina University is gratefully acknowledged.Notes and references
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366 RSC.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed. Engl., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2010, 111, 688–764 CrossRef PubMed.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 CrossRef PubMed.
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- V. Safarifard and A. Morsali, CrystEngComm, 2014, 16, 8660–8663 RSC.
- Z. Zhang and M. J. Zaworotko, Chem. Soc. Rev., 2014, 43, 5444–5455 RSC.
- A. Carne-Sanchez, I. Imaz, K. C. Stylianou and D. Maspoch, Chemistry, 2014, 20, 5192–5201 CrossRef CAS PubMed.
- V. Safarifard and A. Morsali, Coord. Chem. Rev., 2015, 292, 1–14 CrossRef CAS PubMed.
- H. Al-Kutubi, J. Gascon, E. J. R. Sudhölter and L. Rassaei, ChemElectroChem, 2015, 2, 462–474 CrossRef PubMed.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626 RSC.
- U. Mueller, H. Puetter, M. Hesse and H. Wessel, WO 2005/049892, 2005, BASF Aktiengesellschaft.
- A. U. Czaja, N. Trukhan and U. Muller, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- A. Martinez Joaristi, J. Juan-Alcañiz, P. Serra-Crespo, F. Kapteijn and J. Gascon, Cryst. Growth Des., 2012, 12, 3489–3498 CAS.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- M. Hartmann, S. Kunz, D. Himsl, O. Tangermann, S. Ernst and A. Wagener, Langmuir, 2008, 24, 8634–8642 CrossRef CAS PubMed.
- N. Campagnol, T. Van Assche, L. Stappers, J. F. M. Denayer, K. Binnemans, D. E. De Vos and J. Fransaer, ECS Trans., 2014, 61, 25–40 CrossRef PubMed.
- R. Ameloot, L. Stappers, J. Fransaer, L. Alaerts, B. F. Sels and D. E. De Vos, Chem. Mater., 2009, 21, 2580–2582 CrossRef CAS.
- M. Schlesinger, S. Schulze, M. Hietschold and M. Mehring, Microporous Mesoporous Mater., 2010, 132, 121–127 CrossRef CAS PubMed.
- R. Ameloot, L. Pandey, M. Van der Auweraer, L. Alaerts, B. F. Sels and D. E. De Vos, Chem. Commun., 2010, 46, 3735–3737 RSC.
- I. Richter, M. Schubert and U. Mueller, Patent WO, 2007/131955, 2007.
- D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed. Engl., 2004, 43, 5033–5036 CrossRef CAS PubMed.
- Z. Chen, S. Xiang, D. Zhao and B. Chen, Cryst. Growth Des., 2009, 9, 5293–5296 CAS.
- E. A. Ukraintseva, A. Y. Manakov, D. G. Samsonenko, S. A. Sapchenko, E. Y. Semitut and V. P. Fedin, J. Inclusion Phenom. Macrocyclic Chem., 2013, 77, 205–211 CrossRef CAS.
- M. P. Nicolau, P. S. Bárcia, J. M. Gallegos, J. A. Silva, A. E. Rodrigues and B. Chen, J. Phys. Chem. C, 2009, 113, 13173–13179 CAS.
- T. Uemura, Y. Ono, K. Kitagawa and S. Kitagawa, Society, 2008, 87–94 CAS.
- J. Y. Lee, D. H. Olson, L. Pan, T. J. Emge and J. Li, Adv. Funct. Mater., 2007, 17, 1255–1262 CrossRef CAS PubMed.
- J. Y. Lee, L. Pan, X. Huang, T. J. Emge and J. Li, Adv. Funct. Mater., 2011, 21, 993–998 CrossRef CAS PubMed.
- H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chemistry, 2005, 11, 3521–3529 CrossRef CAS PubMed.
- J. Liu, J. Y. Lee, L. Pan, R. T. Obermyer, S. Simizu, B. Zande, J. Li, S. Sankar and J. K. Johnson, J. Phys. Chem. C, 2008, 112, 2911–2917 CAS.
- Y. Chen, J. Lee, R. Babarao, J. Li and J. Jiang, J. Phys. Chem. C, 2010, 114, 6602–6609 CAS.
- I. Senkovska and S. Kaskel, Microporous Mesoporous Mater., 2008, 112, 108–115 CrossRef CAS PubMed.
- Z. Liang, M. Marshall and A. L. Chaffee, Microporous Mesoporous Mater., 2010, 132, 305–310 CrossRef CAS PubMed.
- T. Tsuruoka, S. Furukawa, Y. Takashima, K. Yoshida, S. Isoda and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 48, 4739–4743 CrossRef CAS PubMed.
- M.-H. Pham, G.-T. Vuong, F. d. r.-G. Fontaine and T.-O. Do, Cryst. Growth Des., 2012, 12, 3091–3095 CAS.
- A. Umemura, S. Diring, S. Furukawa, H. Uehara, T. Tsuruoka and S. Kitagawa, J. Am. Chem. Soc., 2011, 133, 15506–15513 CrossRef CAS PubMed.
- I. Stassen, M. Styles, T. Van Assche, N. Campagnol, J. Fransaer, J. Denayer, J.-C. Tan, P. Falcaro, D. De Vos and R. Ameloot, Chem. Mater., 2015, 27, 1801–1807 CrossRef CAS.
- N. Li-yan, H. Rui-nian, N. Gui-ling and O. Xiao-xia, Chem. Res. Chin. Univ., 2012, 28, 555–558 Search PubMed.
- S. Diring, S. Furukawa, Y. Takashima, T. Tsuruoka and S. Kitagawa, Chem. Mater., 2010, 22, 4531–4538 CrossRef CAS.
- M. H. Pham, G. T. Vuong, A. T. Vu and T. O. Do, Langmuir, 2011, 27, 15261–15267 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |