Aptamer-based microcantilever array biosensor for detection of fumonisin B-1†
Abstract
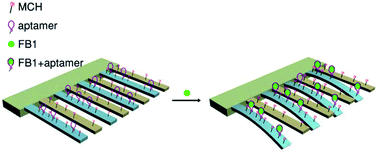
An aptamer-based microcantilever array sensor for detection of fumonisin B-1 (FB1) was developed. The sensing cantilevers in the array were functionalized with self-assembled monolayers (SAMs) of thiolated FB1-specific aptamer, while the reference cantilevers were modified with 6-mercapto-1-hexanol SAMs to eliminate interference from the environment by detecting the deflection induced by nonspecific interactions. This differential deflection amplitude between sensing and reference cantilevers shows a linearity relation with the concentration of FB1 in the range from 0.1 to 40 μg mL−1, with a limit of detection of 33 ng mL−1 (S/N = 3). The sensor exhibits good specificity over ochratoxin A (OTA) and deoxynivalenol (DON). This biosensor provides a promising approach to detect FB1 in food and agricultural products.

 Please wait while we load your content...
Please wait while we load your content...