PEG-assisted hydrothermal synthesis and electrochemical performance of ZnO/Ketjenblack nanocomposite for lithium ion batteries
Chao Chena,
Huang Zhangb,
Yunlong Xu*a,
Mandi Jia,
Hui Donga and
Chongjun Zhaoa
aKey Laboratory for Ultrafine Materials of Ministry of Education, Shanghai Key Laboratory of Advanced Polymeric Materials, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, PR China. E-mail: xuyunlong@ecust.edu.cn; Fax: +86-021-64250838; Tel: +86-021-64252019
bDepartment of Materials Engineering (MTM), KU Leuven, Kasteelpark Arenberg 44, B-3001 Leuven, Belgium
First published on 22nd April 2015
Abstract
ZnO/Ketjenblack(KB) composite was fabricated by means of a facile PEG-assisted hydrothermal synthesis process, characterized by X-ray powder diffraction, scanning electron microscopy, field emission transmission electron microscopy, thermogravimetric analysis, nitrogen sorption, energy dispersion spectroscopy, galvanostastic charge/discharge test, cyclic voltammogram and electrochemical impedance spectroscopies. The results show that the composite forms a special porous structure with ZnO particles embedded in the mesopores of Ketjenblack, which favors the improvement of electrochemical performance. Compared with unmodified ZnO, ZnO/KB composite exhibits superior electrochemical performances. ZnO/KB composite delivers a discharge capacity of 418.9 mA h g−1 at the discharge density current of 800 mA g−1, whereas the ZnO only gives 116.1 mA h g−1. Moreover, the sample retains a discharge capacity of 538.4 mA h g−1 after 100 cycles at a current density of 100 mA g−1. The improved electrochemical performance can be ascribed to the combined Ketjenblack, which serves as conducting buffering matrix during lithiation/delithiation process.
Introduction
Recently, due to the high theoretical capacity of 978 mA h g−1,1 which is two times larger than that of graphite anode (∼370 mA h g−1),2,3 ZnO is attracting more and more attention as alternative anode material to graphite in LIBs. However, severe capacity fading, caused by the intrinsic low conductivity and the pulverization arising from the large volume change during lithiation/delithiation process, hinders its practical application.4,5 Hitherto, a lot of efforts have been devoted to overcoming these problems and many approaches have been adopted. It has been reported that the electrochemical properties of ZnO can be improved by (i) doping and forming composite with metal, metal oxide or carbonaceous materials,6–8 (ii) creating void space in the structure,9 (iii) preparing ordered structured materials,10,11 (iv) downsizing the particle.12Inspired by the above results, downsizing the particle occurs to an efficient way to improve their electrochemical properties. Many approaches have been employed to synthesize ZnO nanostructures, including chemical vapor deposition,13 physical vapor deposition,14 electro deposition15 and hydrothermal process. Among all kinds of adopted methods, the hydrothermal process seems to be a promising aqueous-based precipitation route, allowing control over the nucleation and crystal growth by simply tuning the hydrothermal reaction conditions. Polyethylene glycol (PEG), as a conventional polymer surfactant to control the orientation and dimension of ZnO, has been used to construct diverse morphologies of ZnO during the hydrothermal synthesis process.16,17
Meanwhile, Ketjenblack is a highly conductive carbon with a typically mesoporous structure.18 Using the Ketjenblack to achieve a novel nano/mesoporous structure has already been reported.19–21 Owing to the mesoporous structure and conductive character, Ketjenblack can not only strengthen the electron conduction, but also effectively prevent the pulverization and agglomeration from the volume change during the charge/discharge process, which consequently leads to are markable improvement in cycling capability.
Herein, we reported a facile PEG-assisted hydrothermal method to synthesize the ZnO/KB nanocomposite with an open 3D network structure and the electrochemical performances as the anode material for lithium-ion batteries were investigated.
Experimental section
Sample preparation
All chemicals are of analytical reagent grade and used without further purification. ZnO/KB composites were synthesized by precipitation from aqueous solution under hydrothermal condition in the following way:The first step: Zn(NO3)2·6H2O, (NH4)2CO3 and polyethylene glycol (PEG, molecular weight: 2000) were dissolved in distilled water to form 1.0 M Zn(NO3)2 solution, 1.0 M (NH4)2CO3 solution and 0.4% PEG solution, respectively. Then stoichiometric amounts of zinc nitrate hexahydrate and Ketjenblack EC 300J (Akzonobel) were mixed in 150 mL distilled water with fully constant stirring, then repeatedly filtrated to obtain the precursors. After that, the precursors were immersed with the filtrate to form suspension. With vigorous constant stirring, 20 mL of 1.0 M (NH4)2CO3 solution were added dropwise into the above suspension at 25 °C. Precipitates were collected, and washed with deionized water and ethanol several times, respectively.
The second step: after washed, the resulting precipitates were dispersed in 0.4% PEG (2000) solution and transferred into a Teflon-lined autoclave. After being sealed, the autoclave was put into an oven and maintained at 200 °C for 10 h. After cooling down to room temperature, the deposited ZnO/KB composite were washed with deionized water and allowed drying at 80 °C. As reference, the bulk ZnO particles were hydrothermally synthesized under the same conditions by removing KB.
Sample characterization
X-ray powder diffraction (XRD) patterns were collected by X-ray diffractometer (D/MAX 2550V, Japan) with Cu Kα (k = 1.5406 Å). The morphology and particle structure of as-prepared ZnO sample were characterized by scanning electron microscopy (SEM, S-3400N) and field emission transmission electron microscopy (TEM, JEM-2100, Japan). The thermogravimetric analysis (TGA) was carried out on a SDT-Q600 thermal analyzer to evaluate the carbon content. The Brunauer–Emmett–Teller (BET) surface area and pore size distribution were evaluated on a TriStar 3000 system.Electrochemical measurements
CR2032 coin cells were fabricated in an argon-filled glove box (MIKROUNA) for electrochemical measurements. The synthesized samples were mixed with acetylene black and polyvinylidene fluoride (PVDF) in the weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by blending in N-methyl-2-pyrrolidone (NMP). The resultant slurries were then pasted on copper foil and dried at 120 °C for 12 h under vacuum environment. After drying, the samples were used as anode. Lithium foil was used as both counter electrodes and the electrolyte was 1 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC) (1
1 by blending in N-methyl-2-pyrrolidone (NMP). The resultant slurries were then pasted on copper foil and dried at 120 °C for 12 h under vacuum environment. After drying, the samples were used as anode. Lithium foil was used as both counter electrodes and the electrolyte was 1 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). A Celgard 2400 mesoporous membrane was used as the separator. The cells were aged for 12 h before measurement. The charge/discharge test was carried out with a LAND Battery Program-control Test System (CT2001A,China) over a voltage range of 0.01–3.0 V. Cyclic voltammogram (CV: 0.1 mV s−1, 0.01 ∼ 3 V) tests were conducted on an Electrochemical Workstation (CHI, 660B, CHENHUA, China). Electrochemical impedance spectroscopies (EIS) of the cells were also conducted on the Electrochemical Workstation. The EIS spectra were potentiostatically collected by using a DC potential equal to the open circuit voltage of the cell and an AC oscillation of 5 mV over a frequency range of 105 Hz–0.01 Hz. All tests were performed at room temperature (25 °C).
1 in volume). A Celgard 2400 mesoporous membrane was used as the separator. The cells were aged for 12 h before measurement. The charge/discharge test was carried out with a LAND Battery Program-control Test System (CT2001A,China) over a voltage range of 0.01–3.0 V. Cyclic voltammogram (CV: 0.1 mV s−1, 0.01 ∼ 3 V) tests were conducted on an Electrochemical Workstation (CHI, 660B, CHENHUA, China). Electrochemical impedance spectroscopies (EIS) of the cells were also conducted on the Electrochemical Workstation. The EIS spectra were potentiostatically collected by using a DC potential equal to the open circuit voltage of the cell and an AC oscillation of 5 mV over a frequency range of 105 Hz–0.01 Hz. All tests were performed at room temperature (25 °C).
Results and discussion
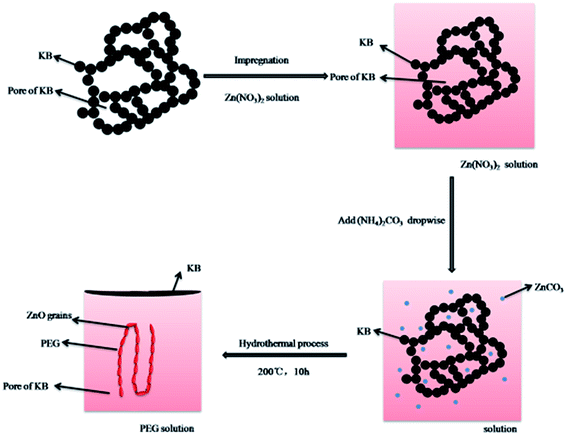
Fig. 1 is the schematic diagram of ZnO/KB composite formation. In the process of ZnO formation, ZnCO3 was formed in the pore of KB with the filtration process as the precursor.22 With the presence of a water-soluble polymer (PEG 2000), which acted as both the catalyst and surfactant,23 zinc oxide crystals form heterogeneous nucleus in the pore and the interface of KB by the dehydration of ZnCO3. Finally, the formation of ZnO/KB composite completes totally after hydrothermal treatment. The nonionic surfactant PEG with a uniform and ordered chain structure has a great influence on the growth process of ZnO.16,17 The small ZnO crystal grains will be enwrapped into the coil of PEG and form agglomerates. According to the theory of crystal growth thermodynamics in the over saturated solution,16 numerous small ZnO grains will be dissolved or recrystallized at a thermodynamic balance state. Only the large crystalline grains, whose size is larger than the critical size, would grow up spontaneously, while the smaller ones under the critical size will re-dissolve into solution. On this level, the PEG coils here just provide assistance to the crystallization of ZnO crystals and the coalescence of adjacent crystalline grains.16 Thus, the PEG provides the active sites for ZnO crystalline growth. As we discussed above, it is well known that the interaction between PEG and zinc species could accelerate the nucleation, and the coiled agglomerate could be in favor of the crystallization of ZnO crystal grains.Shown in Fig. 2 are the XRD patterns of ZnO/KB nanocomposite, ZnO and KB, as well as the JCPDS standard diffraction patterns of ZnO, respectively. All the diffraction peaks of ZnO/KB can be indexed as the featured hexagonal phase of ZnO with the lattice constants a = 3.246 Å, b = 3.231 Å, c = 5.2092 Å, which is in good agreement with the standard data of JCPDS CARD no. 36-1451 (a = 3.250 Å, b = 3.250 Å, c = 5.2071 Å). These results imply the prepared ZnO was well crystallized. This consistency illuminates that the emergence of KB have no effect to the hydrothermal synthesis. Apart from ZnO diffraction peaks, no additional peaks can be detected, mirroring the high purity of the product. Additionally, the slight protuberance between 15° and 30° in the XRD pattern of ZnO/KB can be ascribed to the amorphous KB, which is consistent with the broad peak of KB in the range of 15–30° shown in XRD results.
The carbon content of ZnO/KB was measured by the thermogravimetric (TG) technique under an ambient atmosphere. As shown in Fig. 3, the two weight loss peaks at 71 °C and 320 °C correspond to the removal of physically adsorbed water and the thermal decomposition of KB, respectively. The mass remains unchanged after 500 °C. The weight loss between 320 and 500 °C can be ascribed to the oxidization and subsequent decomposition of KB, and the value can be considered as the carbon content. Hence, the carbon content of the as-obtained ZnO/KB is calculated to be 24.94%.
The nitrogen adsorption–desorption isotherm and pore size distribution curves (inset) of KB and ZnO/KB composite are shown in Fig. 4. According to the IUPAC classification,24 the isotherm shows a typical V isotherm with H1 hysteresis loop for both the KB and the ZnO/KB nanocomposite, indicating the mesoporous structure. The BET surface area, pore volume and pore diameter of the samples are listed in Table 1. As shown in Table 1, the values of the above parameters are 549.54 m2 g−1, 0.69 cm2 g−1, 3.63 nm for the ZnO/KB composite and 1265.92 m2 g−1, 3.01 cm2 g−1, 7.199 nm for KB, respectively. The large surface area of KB was benefit to absorption of PEG, which promoted the nucleation of ZnO. Compared to the KB, the decreased values of ZnO/KB composite can be attributed to the embedding of ZnO nanoparticles in mesopores of KB. The large surface area provides more electrochemical active sites and the porosity can also broaden an effective channel for the diffusion of Li+ and electrons, which is beneficial to improve the electrochemical properties of electrode materials.25
 | ||
| Fig. 4 N2 adsorption–desorption isotherms of KB (a) and ZnO/KB (b). The insets show the corresponding pore size distributions. | ||
| Samples | SBET (m2 g−1) | Vtotal (cm3 g−1) | DPore (Å) |
|---|---|---|---|
| ZnO/KB | 549.54 | 0.69 | 36.30 |
| KB | 1265.92 | 3.01 | 71.99 |
The SEM images of pure ZnO, pristine KB and ZnO/KB are as shown in Fig. 5. Fig. 5(a) and (b) show the morphology of pure ZnO, which is in accordance with the above discussion of the formation of ZnO crystals. ZnO crystals grow up along the chains of PEG to form nanorods22 as showed in Fig. 5(b). Fig. 5(c) and (d) show the structure of pristine KB and ZnO/KB composite, respectively. Compared to porous morphology of pristine KB, the similarly loosely-restacked and porous structures are observed in ZnO/KB composite. This result combined with BET results indicate that the load of ZnO will not change the overall skeletons and porous structure of KB, and ZnO is embedded into the KB skeletons without apparent cluster which brings the pore size change of KB. The mesoporous structure of ZnO/KB will be beneficial to accommodate the volume variations and facilitate the electron transfer/Li+ diffusion.
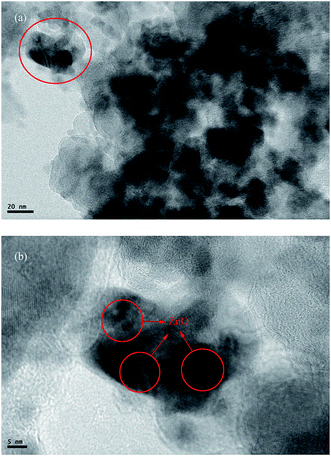
To further confirm the existence of ZnO in the KB, TEM measurement was carried out. The TEM images of ZnO/KB composite are shown in Fig. 6. Fig. 6(b) is the magnification picture of the red circled area in Fig. 6(a). The red circled area was marked to note the area where the lattice fringes appeared. The highly ordered crystalline of ZnO nanoparticles are clearly seen in the red circle in Fig. 6(b), which confirms the existence of ZnO in the pores of KB. Also Fig. 6(b) clearly reveals that ZnO nanoparticles with a diameter of 10 nm are decorated in the mesoporous networks of KB from the lattice fringes. Such structure can efficiently increase the contact area between electrolytes and active ZnO particles, thereby boosting the transportation of lithium-ions.
In order to investigate the uniform dispersion of ZnO nanoparticles in KB, the elemental mapping of ZnO/KB sample was analyzed by Energy Dispersion Spectroscopy (EDS) measurement. Fig. 7(a) shows the SEM image of the tested area, the integral and separate distribution of the ZnO, O and C elements in the tested area is presented in Fig. 7(b). Fig. 7(c–e) exhibit the separate distributions of the different elements O, C and Zn in the area, respectively. As presented in Fig. 6(c and e), the elements ZnO and O have homogeneous distributions, which are rendered as evidence of successful disperse of ZnO in the mesopores of KB through hydrothermal reactions.
Fig. 8(a) displays the cyclic voltammograms (CVs) of ZnO/KB composite and ZnO at a scan rate of 0.1 mV s−1 in the voltage ranging from 0.01 to 3 V. For the electrode of ZnO/KB, in the first cycle, two cathodic peaks are observed at about 0.50 and 0.25 V. The strong reduction peak at 0.25 V is due to the generation of Li–Zn alloy together with the decomposition of electrolyte and the resulting growth of organic-like solid electrolyte interphase (SEI) layer, while the relative weak peak at about 0.5 V is related to a combination of several electrochemical reactions with close potentials, such as the reduction of ZnO into metallic Zn and the formation of amorphous Li2O.10,26,27 During the subsequent anodic scan, there are five different oxidation peaks, located at 0.37, 0.54, 0.69, 1.38 and 2.6 V. The first four can be ascribed to a multi-step de-alloying process of lithium–zinc alloy (LiZn, Li2Zn3, LiZn2 and Li2Zn5) and the decomposition of the organic-like-layer.8,26,28,29 The last peak is found at 2.6 V and it corresponds to the formation of ZnO.30 In the second cycle, the cathodic peak shifts significantly from 0.5 V to a higher potential of 0.74 V while the anodic peak happens lightly. From the second cycle onwards, the CV curves almost superpose in shapes, implying high reversibility of the electrochemical reactions. The CV results of ZnO are smaller than that of ZnO/KB. And the corresponding peaks in ZnO curves are not conspicuous in the CV cycle, due to their smaller specific surface areas and the longer diffusion distances.
According to above results and previous studies, the Li-storage mechanism of ZnO/KB owns to two reversible electrochemical reactions as below:27,31
| ZnO + 2Li+ + 2e− ↔Zn + Li2O | (1) |
| ZnO + xLi+ + xe− ↔ LixZn (x ≤ 1) | (2) |
Fig. 8(b) discloses the initial two charge–discharge curves of ZnO and ZnO/KB-based electrodes at 100 mA g−1 in the voltage range of 0.01–3.0 V (vs. Li+/Li). Two plateaus in the initial discharge curve can be observed. The long flat plateau near 0.5 V corresponds to the reduction of ZnO into Zn as well as the formation of Li2O, while the slight plateau appeared around 0.25 V may be assigned to the decomposition of electrolyte and formation of lithium–zinc alloy, which is coincide with the above CV results.1,12,28
In the second discharge curve, the long plateau is replaced by a slope between 1.1 and 0.3 V, which is similar with other ZnO-based electrodes reported previously.11,27,32 However, no obvious plateaus can be observed in the charge curves.
The initial discharge capacities of ZnO/KB and ZnO are 1346.1 and 997.7 mA h g−1, respectively, much higher than the theoretical value of 978 mA h g−1. The excess capacity might originate from electrolyte decomposition in the low-potential region, formation of solid electrolyte interface (SEI) layer and perhaps abundant specific surface area as well as the good dispersity of the ZnO in KB, which would enhance the surface electrochemical reactivity and improve Li-ion storage capacity.33,34 The second discharge capacities of ZnO/KB and ZnO are 718.7 and 585.2 mA h g−1 since irreversible SEI formation is nearly completed after the first lithium insertion and extraction process.29,35
Fig. 8(c) shows the long-term cycling performances and the corresponding Coulombic efficiencies of the ZnO/KB, ZnO and KB at 100 mA g−1 between 0.01 and 3 V. The top two curves are the Coulombic efficiencies of the ZnO/KB and ZnO, respectively. The initial discharge capacity of ZnO/KB, ZnO and KB are 1206.8, 798.7 and 360.9 mA h g−1. During the first 10th cycles, a large capacity fading can be found in ZnO/KB, while the capacity maintains at about 550 mA h g−1 up to 100 cycles with no further significant fading. After 100 cycles, ZnO/KB, ZnO and KB exhibit a reversibility capacity of 538.4, 185.05 and 216.5 mA h g−1. Furthermore, in order to demonstrate the function of the structure of KB, the capacities of ZnO from ZnO/KB were calculated by eliminating the proportion of capacity from KB, as showed in Fig. 8(c). The capacity contributed by ZnO in ZnO/KB can be calculated as 484.5 mA h g−1 after 100 cycles. Thus the ZnO in the composite shows a relative high reversible capacity and excellent cycling stability in comparison with bulk ZnO. Normally, the repeated volume change during the lithium ions insertion/extraction process results in the particle pulverization and electrode deterioration. Hence, the excellent superior cycle performance of the composite can be overwhelmingly attributed to its porosity created by KB skeletons and mirrored the integrity of the ZnO/KB electrode.
The rate capabilities of all samples were also measured from 100 mA g−1 to 800 mA g−1 and the results are depicted in Fig. 8(d). Compared to pristine ZnO, ZnO/KB displays a superior rate capability. The average reversible capacities of ZnO/KB drop from 701, 587.4, 505.3 to 418.9 mA h g−1 as the current densities increase from 100, 200, 500 to 800 mA g−1. Moreover, after being cycled at high rates, the capacity of ZnO/KB can well recover to 627.8 mA h g−1 once the current density is shifted to the original low value, indicating the excellent reversibility of ZnO/KB electrode.
To further understand the role of KB combination on the electrochemical performance of ZnO anode material, electrochemical impedance spectroscopy (EIS) measurements were performed in a frequency range from 0.01 to 105 HZ and the Nyquist plots are given in Fig. 9(a). It can be seen clearly that the impedance spectrum of ZnO and ZnO/KB were composed of a sloping line in the low frequency range and a depressed semicircle in high frequency. The high frequency intercept at the real axis corresponds to bulk resistance (Re) of the cell, which reflects the electronic conductivity of the electrolyte, separator and electrode. The sloping line in the low frequency region is related to Warburg impedance (W), which is associated with the Li-ions diffusion in the bulk electrode. The Nyquist plots are fitted using an equivalent circuit as shown in the insert of Fig. 9(a), and the derived impedance parameters of Re, Rct (the charger-transfer reaction resistance), W are listed in the Table 2. The Rct value of ZnO/KB is much smaller than that of ZnO, which means the lower charger-transfer resistance of the ZnO/KB.
 | ||
| Fig. 9 (a) EIS spectra of ZnO/KB composite in the frequency range between 0.01 Hz and 100 kHz; (b) the relationship between Z′ and ω−1/2 at low frequency for ZnO and ZnO/KB. | ||
| Samples | Re (Ω) | Rct (Ω) | σw (Ω cm2 s−1) |
|---|---|---|---|
| a Re: electrolyte resistance; Rct: charge transfer resistance; σw: Warburg impedance. | |||
| ZnO | 11.52 | 338.74 | 92.48 |
| ZnO/KB | 3.49 | 116.14 | 19.53 |
The diffusion coefficient values of the lithium ions (DLi+) in the bulk electrode can be calculated from the following eqn (1).37
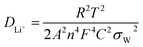 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1), C is the concentration of lithium ion in solid and σw is the Warburg coefficient, ρ is density of the synthesized materials, M is molecular weight of ZnO. The σw can be calculated by the following eqn (2).37
500 C mol−1), C is the concentration of lithium ion in solid and σw is the Warburg coefficient, ρ is density of the synthesized materials, M is molecular weight of ZnO. The σw can be calculated by the following eqn (2).37| Zre = Re + Rct + σwω−1/2 | (2) |
Overall, the enhanced electrochemical performance of ZnO/KB composite can be attributed to its mesoporous structure and extra carbon support. Specifically, KB has a large specific surface area of 822.7 m2 g−1,18 which can provide large contact interfaces between electrode and electrolyte. Moreover, the porosity of KB can also efficiently shorten the Li ion insertion/extraction distance and accommodate the volume change to alleviate the pulverization, resulting in the higher capacity and enhanced rate capability.25,36 Thus, the KB, as a kind of carbonous material, can not only improve the conductivity of electrodes, but also provide extra support to the structures, which plays a positive role in the high reversible capacity and excellent cyclability of the fabricated electrodes.38 These aspects give a result of the improvements of the electrochemical properties of the ZnO/KB composite material.
Conclusions
In summary, ZnO/KB nanocomposite was synthesized through a facile PEG-assisted hydrothermal route. The prepared nanocomposite exhibits a relative high specific capacity, excellent cyclability and high rate capability compared with the pure ZnO. The as-improved performances of ZnO/KB can be attributed to the intrinsic features of KB, namely the mesoporous structure and conductively, which can kinetically advance the ion diffusion in the active materials, efficiently accommodate the huge volume expansion during the discharge/charge processes and significantly enhance the conductivity of active materials. Therefore, the ZnO/KB nanocomposite anode for LIBs achieves significant improvement in reversible capacity and cycle stability over the pure ZnO. This novel and facile method may be extended to prepare other materials for lithium ion batteries.Acknowledgements
The work is supported by Shanghai Nanotechnology Special Foundation (no. 11nm0500900), Shanghai Leading Academic Discipline Project (no. B502) and Shanghai Key Laboratory Project (no. 08DZ2230500).Notes and references
- Q. Pan, L. Qin, J. Liu and H. Wang, Electrochim. Acta, 2010, 55, 5780–5785 CrossRef CAS PubMed.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- H. Buqa, D. Goers, M. Holzapfel, M. E. Spahr and P. Novák, J. Electrochem. Soc., 2005, 152, A474–A481 CrossRef CAS PubMed.
- F. Belliard, P. Connor and J. Irvine, Solid State Ionics, 2000, 135, 163–167 CrossRef CAS.
- H. Li, Z. X. Wang, L. Q. Chen and X. J. Huang, Adv. Mater., 2009, 21, 4593–4607 CrossRef CAS PubMed.
- W. T. Song, J. Xie, S. Y. Liu, Y. X. Zheng, G. S. Cao, T. J. Zhu and X. B. Zhao, Int. J. Electrochem. Sci., 2012, 7, 2164–2174 CAS.
- S. M. Abbas, S. T. Hussain, S. Ali, N. Ahmad, N. Ali and S. Abbas, J. Mater. Sci., 2013, 48, 5429–5436 CrossRef CAS.
- C. Q. Zhang, J. P. Tu, Y. F. Yuan, X. H. Huang, X. T. Chen and F. Mao, J. Electrochem. Soc., 2007, 154, A65–A69 CrossRef CAS PubMed.
- Q. S. Xie, X. Q. Zhang, X. B. Wu, H. Y. Wu, X. Liu and G. H. Yue, Electrochim. Acta, 2014, 125, 659–665 CrossRef CAS PubMed.
- J. P. Liu, Y. Y. Li, X. T. Huang, G. Y. Li and Z. K. Li, Adv. Funct. Mater., 2008, 18, 1448–1458 CrossRef CAS PubMed.
- J. P. Liu, Y. Y. Li, R. M. Ding, J. Jiang, Y. Y. Hu, X. X. Ji, Q. B. Chi, Z. H. Zhu and X. T. Huang, J. Phys. Chem. C, 2009, 113, 5336–5339 CAS.
- K. T. Park, F. Xia, S. W. Kim, S. B. Kim, T. Song, U. Paik and W. I. Park, J. Phys. Chem. C, 2013, 117, 1037–1043 CAS.
- F. Belliard, P. A. Connor and J. T. S. Irvine, Ionics, 1999, 5, 450–454 CrossRef CAS.
- Y. C. Kong, D. P. Yu, B. Zhang, W. Fang and S. Q. Feng, Appl. Phys. Lett., 2001, 78, 407–409 CrossRef CAS PubMed.
- L. F. Xu, Y. Guo, Q. Liao, J. P. Zhang and D. S. Xu, J. Phys. Chem. B, 2005, 109, 13519–13522 CrossRef CAS PubMed.
- Y. J. Feng, M. Zhang, M. Guo and X. D. Wang, Cryst. Growth Des., 2010, 10, 1500–1507 CAS.
- X. F. Zhou, D. Y. Zhang, Y. Zhu, Y. Q. Shen, X. F. Guo, W. P. Ding and Y. Chen, J. Phys. Chem. B, 2006, 110, 25734–25739 CrossRef CAS PubMed.
- J. Y. Choi, R. S. Hsu and Z. W. Chen, J. Phys. Chem. C, 2010, 114, 8048–8053 CAS.
- C. C. Chang, H. K. Su, L. J. Her and J. H. Lin, J. Chin. Chem. Soc., 2012, 59, 1233–1237 CrossRef CAS PubMed.
- X. Yang, Y. L. Xu, H. Zhang, Y. A. Huang, Q. Jiang and C. J. Zhao, Electrochim. Acta, 2013, 114, 259–264 CrossRef CAS PubMed.
- H. Dong, Y. L. Xu, M. D. Ji, H. Zhang, Z. Zhao and C. J. Zhao, Electrochim. Acta, 2015, 151, 118–125 CrossRef CAS PubMed.
- L. J. An, J. Wang, T. F. Zhang, H. L. Yang and Z. H. Sun, Adv. Mater. Res., 2012, 380, 335–338 CrossRef CAS.
- F. Tokiwa and K. Tsujii, Bull. Chem. Soc. Jpn., 1973, 46, 2684–2686 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- A. Vu, Y. Q. Qian and A. Stein, Adv. Energy Mater., 2012, 2, 1056–1085 CrossRef CAS PubMed.
- H. B. Wang, Q. M. Pan, Y. X. Cheng, J. W. Zhao and G. P. Yin, Electrochim. Acta, 2009, 54, 2851–2855 CrossRef CAS PubMed.
- X. H. Huang, X. H. Xia, Y. F. Yuan and F. Zhou, Electrochim. Acta, 2011, 56, 4960–4965 CrossRef CAS PubMed.
- F. Belliard and J. T. S. Irvine, J. Power Sources, 2001, 97–98, 219–222 CrossRef CAS.
- M. S. Wu and H. W. Chang, J. Phys. Chem. C, 2013, 117, 2590–2599 CAS.
- Y. Sharma, N. Sharma, G. V. S. Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS PubMed.
- L. Qiao, X. H. Wang, X. L. Sun, X. W. Li, Y. X. Zheng and D. Y. He, Nanoscale, 2013, 5, 3037–3042 RSC.
- M. Ahmad, Y. Y. Shi, A. Nisar, H. Y. Sun, W. C. Shen, M. Wei and J. Zhu, J. Mater. Chem., 2011, 21, 7723–7729 RSC.
- X. J. Zhu, Y. W. Zhu, S. Murali, M. D. Stoller and R. S. Ruoff, J. Power Sources, 2011, 196, 6473–6477 CrossRef CAS PubMed.
- S. L. Shi, Y. G. Liu, J. Y. Zhang and T. H. Wang, Chin. Phys. B, 2009, 18, 4564–4607 CrossRef CAS.
- Z. W. Fu, F. Huang, Y. Zhang, Y. Chu and Q. Z. Qin, J. Electrochem. Soc., 2003, 150, A714–A720 CrossRef CAS PubMed.
- L. Zhou, D. Y. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745–748 CrossRef CAS PubMed.
- Q. Cao, H. P. Zhang, G. J. Wang, Q. Xia, Y. P. Wu and H. Q. Wu, Electrochem. Commun., 2007, 9, 1228–1232 CrossRef CAS PubMed.
- Z. X. Yang, G. D. Du, Q. Meng, Z. P. Guo, X. B. Yu, Z. X. Chen, T. L. Guo and R. Zeng, J. Mater. Chem., 2012, 22, 5848–5854 RSC.
| This journal is © The Royal Society of Chemistry 2015 |