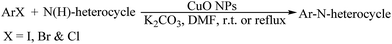
Tamarix gallica leaf extract mediated novel route for green synthesis of CuO nanoparticles and their application for N-arylation of nitrogen-containing heterocycles under ligand-free conditions
Mahmoud Nasrollahzadeh*a,
S. Mohammad Sajadib and
Mehdi Mahamc
aDepartment of Chemistry, Faculty of Science, University of Qom, PO Box 37185-359, Qom, Iran. E-mail: mahmoudnasr81@gmail.com; Fax: +98 25 32103595; Tel: +98 25 32850953
bDepartment of Petroleum Geoscience, Faculty of Science, Soran University, PO Box 624, Soran, Kurdistan Regional Government, Iraq
cDepartment of Chemistry, Aliabad Katoul Branch, Islamic Azad University, Aliabad Katoul, Iran
First published on 29th April 2015
Abstract
We report the green synthesis of CuO nanoparticles (CuO NPs) using Tamarix gallica leaf extract and their catalytic activity for N-arylation of nitrogen-containing heterocycles with aryl halides under ligand-free conditions. Tamarix gallica leaves are used as a bio-material for the first time as reducing agent. The green synthesized CuO NPs was characterized using the powder XRD, TEM, UV-vis and FT-IR. This method offers several advantages, viz. high yields, simple methodology, recyclability of the catalyst and a simple workup procedure.
Introduction
C(ayrl)–N(heterocycle) bond formation is an important organic reaction since the resultant N-arylated heterocyclic product plays an important role in the synthesis of many substances such as drugs, materials, ionic liquids, N-heterocyclic carbenes, natural products and biologically active compounds.1In the past years, a great deal of attention has been focused on the development of metal catalyzed arylation.2 However, these methodologies suffer from numerous limitations such as high reaction temperature (>200 °C), long reaction times, the high cost of catalysts, use of expensive, toxic and sensitive to air ligands, the need for stoichiometric amounts of catalyst and a major challenge lies in the separation of product from expensive catalyst. In addition to these problems, the major drawback is that the majority of catalysts cannot be reused. To overcome from these problems, we have investigated a new and ligand-free catalytic system for N-arylation of nitrogen-containing heterocycles. In contrast with homogeneous catalysts, heterogeneous catalysts are easy to separate and can be recycled. This is very beneficial for industrial process in the green chemistry domain.
Due to high surface-to-volume ratio and metal NPs highly active surface compared to the bulk metals, heterogeneous catalysts are more and more used in the form of NPs.3 Among various heterogeneous nanocatalysts, CuO NPs containing high surface area have received considerable attention because of their good chemical and thermal stability, low cost, low toxicity, ease of handling and potential applications in organic synthesis as an efficient and recyclable catalyst.4 The CuO NPs are attractive both from economic and industrial points of view, as compared to those of the precious metals. Also, according to the thermodynamic calculations in literature,5 among various copper forms in aqueous media such as Cu(OH)2, Cu2O, etc., CuO is thermodynamically more stable.
Various exploitable methods exist for the synthesis of CuO NPs by conventional physical and chemical synthetic procedures. But some of the methods serious drawbacks and limitations such as the use of toxic, expensive chemicals and flammable organic solvents, and formation of toxic by-product make it very difficult to meet the requirements of environmentally friendly chemical processes.6 In addition, during the chemical synthesis, the residuals of some toxic chemicals may adsorb on the surfaces of the NPs, which prevents their application in biomedical equipment. Therefore, the development of simple, highly efficient methodologies for the preparation of NPs remains desirable. In recent years, environmental friendly and green synthesis of metallic nanoparticles (NPs) has attracted tremendous attention in the scientific community, due to the growing environmental contamination caused by the conventional physical and chemical methods.7 The biosynthetic techniques for the preparation of metal NPs has several advantages over chemical synthesis, such as simplicity, cost effectiveness as well as compatibility for biomedical and pharmaceutical applications. Among biological methods for the preparation of metal NPs plant extracts have attracted significant attention, due to easy and simple sampling, and cost effectiveness of this method facilitates the large scale biosynthesis of NPs.7i,7j Plant extracts may act both as reducing agent and stabilizing agents for the synthesis of metal NPs.
The Tamarix gallica from the family of Tamaricaceae is a tree or shrub halophyte from coastal regions and desert, relatively long-living plant that can tolerate a wide range of environmental conditions and resist abiotic stresses such as salt, high temperature, and drought stresses (Fig. 1).8
As a part of our ongoing research on the green synthesis and catalytic applications of various metal NPs in organic reactions,9 we herein report a novel and simple method for the green synthesis of CuO NPs using Tamarix gallica leaf extract as a reducing and stabilizing agent. The CuO NPs were employed as a recyclable catalyst for N-arylation of nitrogen-containing heterocycles with aryl halides under ligand-free conditions (Scheme 1). The N-arylation reaction has been successfully achieved with good to excellent yields. The catalyst exhibited remarkable reusable activity.
Result and discussion
In the present investigation, CuO NPs was synthesized using Tamarix gallica leaf extract. In synthesis of nanoparticles, extracts from plant may act both as reducing and capping agents.The leaves of this species is extensively used as a source of herbal medicine for presence of medicinal phytochemicals. Fig. 2 shows the HPLC fingerprint of leaf extract of the plant demonstrating the presence of potent antioxidants such as gallic acid (1), Epicatechin (2), syringic acid (3), vanillic acid (4), rosmarinic acid (5), p-coumaric acid (6), ferrulic acid (7), quercitin (8), trans-cinnamic acid (9) and flavone (10) respectively. These phyto-constituents confirmed the application of Tamarix gallica leaf extract as a suitable source for synthesis of nanoparticles using the reducing ability of its antioxidant contents.
The UV spectrum of plant extract shows bonds due to the transition localized within the ring of cinnamoyl and benzoyl system. Although, they are generally related the π → π* transitions of double bounds but this absorbent bonds are specifications of flavones nuclei inside the extract as supported by scientific report.10 Therefore, the UV spectrum of extract (Fig. 3) shows bonds at λmax 350 nm (bond I) due to the transition localized within the ring of cinnamoyl system; whereas the one centered at 231 nm (bond II) is for ring related to the benzoyl system. They are related to the π → π* transitions and these absorbent bonds demonstrate the presence of polyphenolics.
In our research there is a focus on the synthesis of NPs in aqueous media using reducing properties of antioxidant phytochemicals inside the plant specially polyphenolics as a major reducing and polyhydroxyl highly polar agents in Tamarix gallica leaf extract according the below possible mechanism (Scheme 2). As indicated in HPLC chromatogram, there are many antioxidant compounds inside the plant extract therefore based on the proposed mechanism Cu(II) are reduced to Cu(0) then because of the highly oxidation potential of Cu(0) to combination with oxygen and reach the higher oxidation states it spontaneously converts to the CuO NPs, Scheme 2, steps 1 and 2.
 | ||
| Scheme 2 Reducing ability of antioxidant phenolics of Tamarix gallica leaf extract to produce nano particles. | ||
As demonstrated using X-ray diffraction analysis, the produced CuO NPs has a crystal structure which the formation of these nanocrystals can be explained via the mechanism briefly as formation of CuO nanocrystals using the coagulation of smaller particles to produce the large nanocrystals through quasi-spherical particles as transition state (Scheme 3). Firstly, the produced CuO NPs undergoes nucleation from a required critical number of nanoparticles in the solvent. Step 3 indicates the growth of this nucleus by diffusion process onto the surface of the nucleated particle cause to produce interparticles; in step 4, interparticles growth by collision and fusion of two particles via the oriented attachment route; and in final step interparticles growth via exchange (dissolution and diffusion) of molecules between various particles, commonly referred to ripening of CuO nanocrystals.11
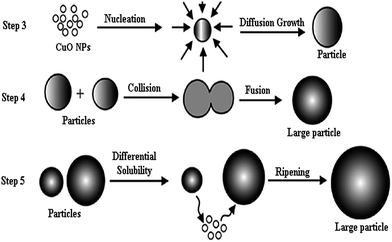 | ||
| Scheme 3 Formation of CuO nanocrystals using the coagulation of smaller particles to produce the large nanocrystals through quasi-spherical particles as transition state. | ||
The green synthesized CuO NPs was characterized by X-ray diffraction (XRD), transmission electron microscope (TEM), FT-IR and UV-vis techniques.
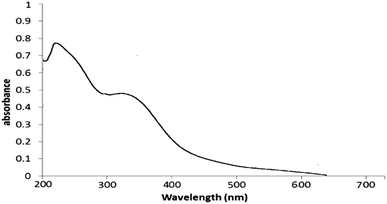
The formation of CuO NPs was controlled by UV-vis spectroscopy. Fig. 4 shows the UV-vis spectrum of CuO NPs formation. The reaction was completed after 3 min. Our results showed that the maximum absorbance of green synthesized CuO NPs was due to the surface plasmon absorption of nanosized cupric oxide particles. Changing the color of the reaction (yellow to dark brown, Fig. 5) and appearance the maxima at 250 nm indicates the reduction process and formation of nanoparticles. As monitored by UV-vis, the synthesized CuO NPs by this method are approximately stable with a few variances in the shape, position and symmetry of the absorption peak even after one month which indicates the relative stability of product.
 | ||
| Fig. 4 UV-vis spectrum of green synthesized CuO NPs using the aqueous extract of leaves of the Tamarix gallica in 3 min to one month. | ||
 | ||
| Fig. 5 Photograph of Tamarix gallica leaf extract (A) and green synthesized CuO NPs produced after bioreaction (B). | ||
The FT-IR analysis was carried out to identify the possible biomolecules responsible for the reduction of Tamarix gallica and CuO NPs. The FT-IR spectrum of the crude extract (Fig. 6) depicted some peaks at 3393, 2925, 1661, 1442, 1300 to 1000 cm−1 which represent free OH in molecule and OH group forming hydrogen bonds, saturated hydrocarbons (Csp3–H), carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), stretching C
O), stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring and C–OH stretching vibrations, respectively. Because of presence the mentioned functional groups inside the structure of polyphenolics, the spectrum can demonstrate the presence of phenolics in the plant leaf extract. The presence of phenolics in the extract could be probably responsible for the reduction of metal ions and formation of nanoparticles.
C aromatic ring and C–OH stretching vibrations, respectively. Because of presence the mentioned functional groups inside the structure of polyphenolics, the spectrum can demonstrate the presence of phenolics in the plant leaf extract. The presence of phenolics in the extract could be probably responsible for the reduction of metal ions and formation of nanoparticles.
Furthermore, FT-IR spectrum of CuO NPs is shown in Fig. 7. The appeared bands are due to lattice vibration modes indicating the functional groups of samples. The broad band in 3305 cm−1 is OH stretching bond. The band around 1435 cm−1 is generally attributed to the bending vibration of sp2-carbon groups for aromatic and 1622 cm−1 for carbonyl functional group.
The X-ray diffraction analysis of prepared CuO NPs was carried to identify the product. The XRD spectrum shown in Fig. 8 contains all the peaks associated with the crystalline planes of pure CuO confirming the crystallinity and phase purity of the NPs.12 No other diffraction peaks arising from metallic Cu or Cu2O present in the XRD pattern, which indicate the high phase purity of synthesized sample.
 | ||
| Fig. 8 XRD powder pattern of CuO NPs synthesized using aqueous extract of leaves of Tamarix gallica. | ||
TEM studies of the CuO NPs were carried out to investigate the shape and the size of the particles. A typical TEM image of CuO NPs is shown in Fig. 9. The image clearly shows the formation of CuO NPs of different sizes and shapes, mainly spherical. The particles are of various sizes.
Evaluation of the catalytic activity of CuO NPs through the N-arylation of nitrogen-containing heterocycles
Usually the ligands play an important role for a successful copper-catalyzed C–N coupling reaction. Most of these ligands are air and moisture sensitive, difficult to prepare, and expensive. However, CuO NPs-catalyzed N-arylation reaction under ligand-free conditions is a topic of considerable interest because of both economic and environmental reasons.Initially, we chose to study the reaction between iodobenzene (3.3 mmol) and 1,2,4 triazoles (3.0 mmol) to optimize the reaction conditions (Table 1). The effect of catalyst loading, base and solvent on the model N-arylation reaction was investigated. Control experiments show that there is no reaction in the absence of catalyst or base. The effect of reaction solvent was also investigated. According to data given in Table 1, DMF was the most efficient solvent for this reaction (Table 1, entry 6). After choosing DMF as the solvent, we examined several different bases. Among the solvents examined, K2CO3 was found to be the most effective. No significant improvement on the yield was observed with using higher amounts of the catalyst (entry 13). Thus, the optimized reaction conditions for this N-arylation reaction of 1,2,4 triazoles are the CuO NPs (0.2 mmol) in 10 mL of DMF using K2CO3 (3.0 mmol) as base at room temperature under ligand-free conditions (Table 1, entry 6).
| Entry | CuO NPs (mmol) | Solvent | Base | Reaction time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: 3.3 mmol of iodobenzene, 3.0 mmol of 1,2,4 triazoles, 3.0 mmol of base and 10 mL of solvent, room temperature.b Isolated yield. | |||||
| 1 | 0.0 | MeCN | K2CO3 | 12 | 0.0 |
| 2 | 0.2 | MeCN | — | 12 | 0.0 |
| 3 | 0.2 | MeCN | K2CO3 | 12 | 45 |
| 4 | 0.2 | Toluene | K2CO3 | 12 | 71 |
| 5 | 0.2 | MeOH | K2CO3 | 12 | 28 |
| 6 | 0.2 | DMF | K2CO3 | 2 | 92 |
| 7 | 0.2 | DMF | NaOH | 5 | 38 |
| 8 | 0.2 | DMF | Cs2CO3 | 5 | 48 |
| 9 | 0.2 | DMF | DBU | 12 | 0.0 |
| 10 | 0.2 | DMF | Et3N | 12 | 21 |
| 11 | 0.2 | DMF | t-BuOK | 5 | 58 |
| 12 | 0.2 | DMF | K2CO3 | 10 | 92 |
| 13 | 0.3 | DMF | K2CO3 | 2 | 91 |
With having the optimum conditions in hand, we examined the scope of this reaction with a series of 1,2,4 triazoles. According to the results summarized in Table 2, in all cases, this protocol afforded the desired products in good to excellent yields. As shown in Table 2, reaction of aryl halides with other nitrogen-containing heterocycles afforded the corresponding N-arylated products in good to excellent yield under ligand-free conditions.
| Entry | Het-NH | Aryl halide | Product | Temperature/time (h) | Yieldb (%) | Natureref. |
|---|---|---|---|---|---|---|
| a Reaction condition: 3.3 equiv. of aryl halide, 3.0 equiv. of Het-NH, 0.2 mmol of catalyst, 3.0 equiv. of K2CO3 and 10 mL of DMF.b Isolated yield. | ||||||
| 1 |  |
 |
 |
r.t./4 | 92 | Oil13a |
| 2 |  |
 |
 |
r.t./4 | 82 | Solid, M.P.: 70–72 °C (Lit.13b: 71 °C) |
| 3 |  |
 |
 |
r.t./4 | 93 | Solid, M.P.: 171–173 °C (Lit.13a: 171–173 °C) |
| 4 |  |
 |
 |
r.t./6 | 78 | Solid, M.P.: 171–173 °C (Lit.13a: 171–173 °C) |
| 5 |  |
 |
 |
r.t./6 | 60 | Solid, M.P.: 171–173 °C (Lit.13a: 171–173 °C) |
| 6 |  |
 |
 |
Reflux/5 | 91 | Oil13a |
| 7 |  |
 |
 |
Reflux/5 | 87 | Oil13a |
| 8 |  |
 |
 |
Reflux/5 | 97 | Oil13a |
| 9 |  |
 |
 |
Reflux/5 | 97 | Solid, M.P.: 86–88 °C (Lit.13c: 85–87 °C) |
| 10 |  |
 |
 |
Reflux/5 | 96 | Oil13d |
| 11 |  |
 |
 |
Reflux/5 | 95 | Oil13d |
| 12 |  |
 |
 |
Reflux/5 | 95 | Solid, M.P.: 129–130 °C (Lit.13g: 128–129 °C) |
| 13 |  |
 |
 |
Reflux/6 | 88 | Solid, M.P.: 129–130 °C (Lit.13g: 128–129 °C) |
| 14 |  |
 |
 |
Reflux/6 | 61 | Solid, M.P.: 129–130 °C (Lit.13g: 128–129 °C) |
The present method offers several notable features, compared with the other literature works13 on the N-arylation of nitrogen-containing heterocycles, i.e., (1) the use of plant extract as an economic and effective alternative represents an interesting, fast and clean synthetic route for the large scale synthesis of CuO NPs without use of toxic, hazardous and dangerous materials or surfactant template, (2) avoidance of the toxic ligands and homogeneous catalysts for the N-arylation reactions, (3) wide substrate scope and generality, (4) higher yields, (5) in our method, because of the heterogeneous nature of the CuO NPs catalyst, it can be easily recovered and reused and (6) according to the UV-vis results, the synthesized CuO NPs by this method are quite stable and can be kept for one month.
Catalyst recyclability
For a heterogeneous catalyst, it is very important to examine its ease of separation, recoverability, and reusability especially for commercial applications. Therefore, the recovery and reusability studies of the catalyst were done by conducting the reaction of iodobenzene with 1,2,4 triazoles (Fig. 10). The catalyst could be easily separated from the reaction mixture by mild centrifugation and washed with distilled water. After being air-dried, it can be reused directly without further purification. The recovered catalyst was used in the next run, and almost consistent activity was observed for 5 consecutive cycles. Based on our results described above one can conclude that CuO NPs is an active and stable catalyst during the N-arylation of nitrogen-containing heterocycles with aryl halides. To check the heterogeneity of this catalyst, which is a very important factor, the filtrate of each cycle was analyzed by an ICP technique. ICP analysis was used to examine the possibility of Cu leaching from the catalyst into the solution under the conditions used but no Cu leaching was detected. The absence of copper traces in the solution confirmed that the reaction was heterogeneous and occurring in the surface of catalyst and there was no leaching of metal species involved during the reaction.Conclusion
In conclusion, we have developed an economically and environmentally benign, fast, efficient and safe procedure for the green synthesis of CuO NPs using Tamarix gallica leaf extract without use of toxic, hazardous and dangerous materials or surfactant template. In addition, the catalytic activity of CuO NPs for N-arylation of nitrogen-containing heterocycles with aryl halides under ligand-free conditions was also studied. This methodology offers the competitiveness of recyclability of the catalyst without significant loss of catalytic activity, and the catalyst could be easily recovered and reused several times, thus making this procedure environmentally more acceptable.Experimental
High-purity chemical reagents were purchased from the Merck and Aldrich chemical companies. All materials were of commercial reagent grade. Melting points were determined in open capillaries using a BUCHI 510 melting point apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance DRX-400 spectrometer at 400 and 100 MHz, respectively. FT-IR spectra were recorded on a Nicolet 370 FT/IR spectrometer (Thermo Nicolet, USA) using pressed KBr pellets. The element analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer carried out on Perkin-Elmer 240c analyzer. X-ray diffraction (XRD) measurements were carried out using a Philips powder diffractometer type PW 1373 goniometer (Cu Kα = 1.5406 Å). The scanning rate was 2° min−1 in the 2θ range from 10 to 80°. The shape and size of CuO NPs crystals were identified by transmission electron microscope (TEM) using a Philips EM208 microscope operating at an accelerating voltage of 90 kV. Ultraviolet-visible (UV-vis) absorption spectra were recorded by a Shimadzu UV 2100 PC UV-visible spectrophotometer.Preparation of Tamarix gallica leaf extract
100 g of dried leaves powdered of Tamarix gallica was added to 500 mL double distillated water in 1000 mL flask and well mixed. The preparation of extract was done by using magnetic heating stirrer at 70 °C for 30 min. The obtained extract was centrifuged in 6500 rpm and filtered then filtrate was kept at refrigerator to use further.Preparation of CuO nanoparticles using the aqueous extract of leaves of Tamarix gallica
In a typical synthesis of CuO NPs, 50 mL of Tamarix gallica leaf extract was added drop wise to 50 mL of well-mixed 0.003 M aqueous solution of CuCl2 with constant stirring at 70 °C. After 3 min the color of the solution was changed from yellow to dark brown during the heating process due to excitation of surface plasmon resonance which indicates the formation of CuO NPs and hydrogen donation activity of antioxidants inside the plant. Furthermore, the stability of CuO NPs was monitored by UV-vis spectroscopy at the times ranging 3 min to 30 days. The obtained precipitation was washed three times with absolute ethanol to remove impurities and then dried for 24 h at room temperature.General procedure for N-arylation of nitrogen-containing heterocycles
To a mixture of catalyst (0.2 mmol), aryl halide (3.3 mmol) and Het-NH (3.0 mmol) in DMF (10 mL), K2CO3 (3.0 mmol) was added and the mixture was vigorously stirred at room temperature or under reflux conditions for the appropriate time (Table 2). After completion (as monitored by TLC), the reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (2 × 20 mL). Aqueous layer was centrifuged to recover CuO NPs and the combined organic layers were washed with brine and dried with anhydrous MgSO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel with ethyl acetate: petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as eluent to yield analytically pure product. All products are known in the literature and were characterized by FT-IR, NMR and melting points and their spectroscopic data identical to that reported in the literature.13
9) as eluent to yield analytically pure product. All products are known in the literature and were characterized by FT-IR, NMR and melting points and their spectroscopic data identical to that reported in the literature.13
Acknowledgements
We gratefully acknowledge the Iranian Nano Council and the University of Qom for the support of this work.References
- (a) T. Wiglenda, I. Ott, B. Kircher, P. Schumacher, D. Schuster, T. Langer and R. Gust, J. Med. Chem., 2005, 48, 6516 CrossRef CAS PubMed; (b) Z. M. Jin, Nat. Prod. Rep., 2005, 22, 196 RSC; (c) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (d) T. Wiglenda and R. Gust, J. Med. Chem., 2007, 50, 1475 CrossRef CAS PubMed; (e) D. G. Hulcoop and M. Lautens, Org. Lett., 2007, 9, 1761 CrossRef CAS PubMed; (f) C. Xie, Y. Zhang, Z. Huang and P. Xu, J. Org. Chem., 2007, 72, 5431 CrossRef CAS PubMed.
- (a) M. Schnürch, R. Flasik, A. F. Khan, M. Spina, M. D. Mihovilovic and P. Stanetty, Eur. J. Org. Chem., 2006, 3283 CrossRef PubMed; (b) K. Okano, H. Tokuyama and T. Fukuyama, Org. Lett., 2003, 5, 4987 CrossRef CAS PubMed; (c) G. Y. Cho, P. Remy, J. Jansson, C. Moessner and C. Bolm, Org. Lett., 2004, 6, 3293 CrossRef CAS PubMed; (d) L. B. Zhu, P. Guo, G. C. Li, J. B. Lan, R. G. Xie and J. S. You, J. Org. Chem., 2007, 72, 8535 CrossRef CAS PubMed; (e) L. B. Zhu, G. C. Li, L. Luo, P. Guo, J. B. Lan and J. S. You, J. Org. Chem., 2009, 74, 2200 CrossRef CAS PubMed; (f) K. Hirano, A. T. Biju and F. Glorius, J. Org. Chem., 2009, 74, 9570 CrossRef CAS PubMed; (g) J. W. W. Chang, X. Xu and P. W. H. Chan, Tetrahedron Lett., 2007, 48, 245 CrossRef CAS PubMed; (h) D. L. Guo, H. Huang, Y. Zhou, J. Y. Xu, H. L. Jiang, K. X. Chen and H. Liu, Green Chem., 2010, 12, 276 RSC; (i) Z.-L. Xu, H.-X. Li, Z.-G. Ren, W.-Y. Du, W.-C. Xu and J.-P. Lang, Tetrahedron, 2011, 67, 5282 CrossRef CAS PubMed.
- (a) M. Nasrollahzadeh, F. Babaei, S. M. Sajadi and A. Ehsani, Spectrochim. Acta, Part A, 2014, 132, 423 CrossRef CAS PubMed; (b) M. Nasrollahzadeh, M. Maham and M. M. Tohidi, J. Mol. Catal. A: Chem., 2014, 391, 83 CrossRef CAS PubMed; (c) M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni and M. Khalaj, RSC Adv., 2014, 4, 43477 RSC; (d) M. Nasrollahzadeh, New J. Chem., 2014, 38, 5544 RSC; (e) M. Nasrollahzadeh, B. Jaleh and A. Ehsani, New J. Chem., 2015, 39, 1148 RSC.
- (a) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., 2007, 46, 5583 CrossRef CAS PubMed; (b) L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397 CrossRef CAS PubMed; (c) P. Fakhri, B. Jaleh and M. Nasrollahzadeh, J. Mol. Catal. A: Chem., 2014, 383–384, 17 CrossRef CAS PubMed; (d) Y. He, Mater. Res. Bull., 2007, 42, 190 CrossRef CAS PubMed.
- (a) L. M. Gedansky, E. M. Woolley and L. G. Hepler, J. Chem. Thermodyn., 1970, 2, 561 CrossRef CAS; (b) U. Bertocci and D. D. Wagman, Int. Union Pure Appl. Chem., Marcel Dekker, New York, pp. 287–293 Search PubMed; (c) M. W. Chase Jr, C. A. Davies Jr. J. R. Downey, D. J. Frurip, R. A. McDonald and A. N. Syverud, JANAF Thermochemical tables, 3rd edn, 1985, pp. 1–1856, J. Phys. Chem., Ref. data, 14, Suppl. no. 1 Search PubMed; (d) O. Kubaschewski, C. B. Alcock and P. J. Spencer, Materials thermochemistry, Pergamon Press, Oxford, 6th edn, 1993 Search PubMed; (e) H. Dai, C. K. Thai, M. Sarikaya, F. Baneyx and D. T. Schwartz, Langmuir, 2004, 20, 3483 CrossRef CAS.
- (a) T. M. D. Dang, T. T. T. Le, E. Fribourg-Blanc and M. C. Dang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2011, 2, 6 Search PubMed; (b) A. Sarkar, T. Mukherjee and S. Kapoor, J. Phys. Chem. C, 2008, 112, 3334 CrossRef CAS; (c) H. Zhu, C. Zhang and Y. Yin, J. Cryst. Growth, 2004, 270, 722 CrossRef CAS PubMed.
- (a) E. S. Abdel-Halim, M. H. El-Rafie and S. S. Al-Deyab, Carbohydr. Polym., 2011, 85, 692 CrossRef CAS PubMed; (b) A. R. Jasbi, Phytochemistry, 2006, 67, 1977 CrossRef PubMed; (c) S. P. Dubey, M. Lahtinen and M. Sillanpa, Process Biochem., 2010, 45, 1065 CrossRef CAS PubMed; (d) G. Zhan, J. Huang, M. Du, I. Abdul-Rauf, Y. Ma and Q. Li, Mater. Lett., 2011, 65, 2989 CrossRef CAS PubMed; (e) X. Huang, H. Wu, S. Pu, W. Zhang, X. Liao and B. Shi, Green Chem., 2011, 13, 950 RSC; (f) P. Raveendran, J. Fu and S. L. Wallen, Green Chem., 2006, 8, 34 RSC; (g) K. B. Narayanan and N. Sakthivel, Adv. Colloid Interface Sci., 2010, 156, 1 CrossRef CAS PubMed; (h) S. Rashmi and V. Preeti, Bioresour. Technol., 2009, 100, 501 CrossRef PubMed; (i) S. Iravani, Green Chem., 2011, 13, 2638 RSC; (j) Z. Wang, C. Fang and M. Megharaj, ACS Sustainable Chem. Eng., 2014, 2, 1022 CrossRef CAS.
- (a) S. Drabu, S. Chaturvedi and M. Sharma, Asian J. Pharm. Clin. Res., 2012, 5, 17 Search PubMed; (b) L. Weiqiang, M. Ajmal Khan, X. Zhang and X. Liu, Pak. J. Bot., 2010, 42, 4133 Search PubMed.
- (a) M. Nasrollahzadeh, F. Babaei, P. Fakhri and B. Jaleh, RSC. Adv., 2015, 5, 10782 RSC; (b) M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartouni, M. Bagherzadeh and R. Safari, J. Mol. Catal. A: Chem., 2015, 400, 22 CrossRef CAS PubMed; (c) M. Nasrollahzadeh, S. M. Sajadi and M. Khalaj, RSC Adv., 2014, 4, 47313 RSC; (d) M. Nasrollahzadeh, S. M. Sajadi, M. Maham and A. Ehsani, RSC Adv., 2015, 5, 2562 RSC; (e) M. Nasrollahzadeh, M. Maham, A. Ehsani and M. Khalaj, RSC Adv., 2014, 4, 19731 RSC; (f) M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartouni and M. Bagherzadeh, J. Colloid Interface Sci., 2015, 448, 106 CrossRef CAS PubMed; (g) M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni and M. Khalaj, J. Mol. Catal. A: Chem., 2015, 396, 31 CrossRef CAS PubMed.
- S. V. Bhat, B. A. Naga Sampagi and M. Sivakumar, Chemistry of natural products, Narosa publishing house, New delhi, 2005, p. 585 Search PubMed.
- A. Layek, G. Mishra, A. Sharma, M. Spasova, S. Dhar, A. Chowdhury and R. Bandyopadhyaya, J. Phys. Chem. C, 2012, 116, 24757 CAS.
- R. Sivaraj, P. K. S. M. Rahman, P. Rajiv, S. Narendhran and R. Venckatesh, Spectrochim. Acta, Part A, 2014, 129, 255 CrossRef CAS PubMed.
- (a) N. V. Suramwar, S. R. Thakare and N. T. Khaty, Org. Chem. Int., 2012, 515092 Search PubMed; (b) M. Tenne, S. Metz, I. Munster, G. Wagenblast and T. Strassner, Organometallics, 2013, 32, 6257 CrossRef CAS; (c) Y. Liu, W. Yan, Y. Chen, J. L. Petersen and X. Shi, Org. Lett., 2008, 10, 5389 CrossRef CAS PubMed; (d) R. Xiao, H. Zhao and M. Cai, Tetrahedron, 2013, 69, 5444 CrossRef CAS PubMed; (e) M. Kuil, E. K. Bekedam, G. M. Visser, A. van den Hoogenband, J. W. Terpstra, P. C. J. Kamer, P. W. N. M. van Leeuwen and G. P. F. van Strijdonck, Tetrahedron Lett., 2005, 46, 2405 CrossRef CAS PubMed; (f) A. Khalil, A. Fihri, M. Jouiad and R. Hashaikeh, Tetrahedron, 2014, 55, 5973 CrossRef CAS PubMed; (g) L.-G. Lee, J.-E. Won, M.-J. Kim, S.-E. Park, K.-J. Jung, B.-R. Kim, S. G. Lee and Y.-J. Yoon, J. Org. Chem., 2009, 74, 5675 CrossRef PubMed; (h) N. V. Suramwar, S. R. Thakare, N. N. Karade and N. T. Khaty, J. Mol. Catal. A: Chem., 2012, 359, 28 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |