Unexpected surface superparamagnetism in antiferromagnetic Cr2O3 nanoparticles†
Zhaolong Yanga,
Jing Zhanga,
Daqiang Gaoa,
Zhonghua Zhub,
Guijin Yangc and
Desheng Xue*a
aKey Laboratory for Magnetism and Magnetic Materials of MOE, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: xueds@lzu.edu.cn; Fax: +86-0931-8914160; Tel: +86-0931-8912237
bHunan University of Science and Technology, Xiangtan 411201, P. R. China
cCollege of Physics and Electronic Engineering, Northwest Normal University, Lanzhou 730070, P. R. China
First published on 11th May 2015
Abstract
We report an unexpected superparamagnetic behavior of antiferromagnetic Cr2O3 nanoparticles. The Cr2O3 particle cores retain their original antiferromagnetic phase, while the surfaces of the particles become superparamagnetic. The X-ray diffraction results confirm that the sample has a corundum structure without any other phases. Through X-ray photoelectron spectroscopy characterization, the particle surfaces present three different oxidation states: Cr3+ (antiferromagnetic), Cr4+ (ferromagnetic), and Cr6+ (nonferromagnetic). A bimagnetic particle model with Cr3+ cores and higher Cr oxidation surface states is used to explain the experimental results. In addition, we observe that spin-flop transitions occur in the antiferromagnetic cores below the Néel temperature (292 K). The spin-flop transition field is uncommon compared with other research, this novel behavior is attributed to the presence of superparamagnetism in the surfaces through the exchange field. These findings reveal the significance of surface states in mediating the magnetic properties in antiferromagnetic materials.
1 Introduction
Due to their important applications in exchange bias and spin-valve devices, antiferromagnetic (AF) oxides have been intensively investigated.1–3 Special types of bimagnetic particles with core–shell structures receive more attention: particles where each CrO2 particle is enclosed by a Cr2O3 layer, which is unexpected for ferromagnetic (FM)/AF core–shell structured particles, have been reported;4 the magnetic properties of an AF MnO core and a FM passivation shell (γ-Mn2O3 or Mn3O4) have been investigated;5 the core behaves as a regular antiferromagnet and the shell becomes a two-dimensional diluted antiferromagnet in Co3O4 nanowires;6 in NiO nanoparticles, the core retains its original AF order while the shell behaves as a spin glass system (Ni3+ ions).7,8Cr2O3, as a typical AF material, has received increased attention because of its abundant magnetic properties. Below the Néel temperature (TN = 308 K)4,9,10 in zero magnetic field, the Cr3+ spins align antiferromagnetically along the [111] easy axis.7 Because of the AF order of the spins, the crystal loses both space- and time-reversal symmetry below TN, this symmetry reduction leads to that in sufficiently high magnetic fields along the z axis the spins flop into the basal plane maintaining their AF order, which is called spin-flop (SF). SF is a first-order transition that occurs at a critical field (HSF; HSF = 5.8 T below 90 K).9 Up to now, most groups had observed the SF transition experimentally at sufficiently high magnetic fields in a low temperature range;10,11 among those, Foner et al. systematically investigated the HSF of Cr2O3 and (Cr2O3)0.9·(Al2O3)0.1 from 4.2 K to about 0.95TN,12 which significantly broadened the temperature range where the SF transition happens compared with other experimental or theoretical conclusions.
In this paper, we report an unexpected superparamagnetic (SPM) behavior of AF Cr2O3 nanoparticles, in which the SPM signals could even exist above TN. The magnetic properties of the bimagnetic nanoparticles with AF cores and SPM surface states are studied and the origin of the anomalous magnetic behavior is discussed in detail. More interestingly, the presence of SPM surface state influences the AF core, which means that the behavior of HSF in the AF phase is unusual compared with other research.9–12
2 Experimental section
2.1 Synthesis of the Cr2O3 nanoparticles
The chemical reagent used as the starting material was analytical grade and was used without any further treatment. Cr2O3 nanoparticles were synthesized by thermal decomposition of chromium chloride hexahydrate (CrCl3·6H2O). The starting material was sintered at 800 °C for 1 h in air to obtain the sample. The as-prepared Cr2O3 nanoparticles were annealed in an oxygen atmosphere (Cr2O3–O2) at 500 °C for 1 h to allow study of the origin of the magnetism.2.2 Characterization
X-ray diffraction (XRD; X’Pert PRO PHILIPS with CuKα radiation, Almelo, Netherlands) was employed to study the structure of the sample. The morphology was characterized using scanning electron microscopy (SEM; Hitachi S-4800, Chiyoda-ku, Japan). The microstructure of the sample was investigated using transmission electron microscopy (TEM; Tecnai TMG2F30, FEI) and a high-resolution TEM (HRTEM) microscope equipped with an energy dispersive X-ray spectroscopy (EDX) spectrometer. Measurements of the magnetic properties were performed using a Quantum Design MPMS magnetometer based on a superconducting quantum interference device (SQUID; Quantum Design, Inc., San Diego, USA). X-ray photoelectron spectroscopy (XPS; VG Scientific ESCALAB-210 spectrometer, East Grinstead, UK) was utilized to determine the bonding characteristics and the composition of the nanoparticles.3 Results and discussion
The XRD pattern of the Cr2O3 nanoparticles is shown in Fig. 1. The results indicate that the sample has a corundum structure [rhombohedral lattice system with space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c(167), JCPDS card no. 84-1616, [a = b = 4.97 Å, c = 13.67 Å]] without any other phases. The SEM images (Fig. 2) reveal that the sample is formed of aggregated microplates with a 0.2–0.4 μm thickness and 1–4 μm radius. In order to see the size of the crystallites aggregated into microplates, the sample was measured using TEM after a longtime ultrasonic treatment. As shown in Fig. 3(a), the sample dispersed into small particles after ultrasonic treatment. The particle diameters are distributed in the range of 60–80 nm. On the other hand, using the Scherrer formula for the full width at half-maximum of the main peaks in the XRD pattern, the average crystallite size is calculated to be 68.3 ± 3.9 nm which matches the particle size read from Fig. 3(a) well. From the HRTEM image shown in Fig. 3(b), a clear atom arrangement can be found inside of the particle, and the representative interplanar spacing is 0.25 nm which is equal to the (110) plane of Cr2O3. Fig. 3(c) depicts the corresponding EDX results for our sample, only the elements Cr, O, Cu, and C are present. It can be understood that Cu and C are from the carbon membranes which hold the sample during measurement. Besides, no impurity elements were detected.
c(167), JCPDS card no. 84-1616, [a = b = 4.97 Å, c = 13.67 Å]] without any other phases. The SEM images (Fig. 2) reveal that the sample is formed of aggregated microplates with a 0.2–0.4 μm thickness and 1–4 μm radius. In order to see the size of the crystallites aggregated into microplates, the sample was measured using TEM after a longtime ultrasonic treatment. As shown in Fig. 3(a), the sample dispersed into small particles after ultrasonic treatment. The particle diameters are distributed in the range of 60–80 nm. On the other hand, using the Scherrer formula for the full width at half-maximum of the main peaks in the XRD pattern, the average crystallite size is calculated to be 68.3 ± 3.9 nm which matches the particle size read from Fig. 3(a) well. From the HRTEM image shown in Fig. 3(b), a clear atom arrangement can be found inside of the particle, and the representative interplanar spacing is 0.25 nm which is equal to the (110) plane of Cr2O3. Fig. 3(c) depicts the corresponding EDX results for our sample, only the elements Cr, O, Cu, and C are present. It can be understood that Cu and C are from the carbon membranes which hold the sample during measurement. Besides, no impurity elements were detected.
 | ||
| Fig. 2 (a and b) SEM images of the Cr2O3 nanoparticles. The nanoparticles aggregate to form microplates. | ||
Fig. 4(a) shows the zero-field-cooled (ZFC) and field-cooled (FC) magnetization curves. It is worth noting that two temperature peaks were observed: the first broad one (Tp1) at around 91 K and the second one (Tp2) at about 292 K. When cooling down from 330 K, the ZFC and FC curves overlap closely until a peak at 292 K appears in both curves. Then they follow a similar trend, that is, the susceptibility increases slowly with the decreasing temperature. Below Tp1, the dividable curves reveal that the magnetization reversal is irreversible. We also obtained magnetization curves as a function of applied magnetic field (M–H) at different temperatures [Fig. 4(b)–(e)]. The curves after deducting the AF contribution are shown in Fig. S3.† It can be seen that all the M–H curves show an S-shaped signal in lower fields (<0.5 T), and the curves measured below Tp2 show a nonlinear behavior with the increasing of the magnetization in higher fields (>2 T). The latter curves are characteristic of SF transitions. In order to further study the SF transition, we have obtained the numerical derivative of the M–H curves, and HSF is defined as the maximum in the derivative curves in the Fig. 4(f)–(i). The SF transition is observed from 20 K to 250 K and HSF decreases firstly, then increases with the rise of the measurement temperature: the corresponding HSF is 5.1, 4.3, and 6.1 × 104 Oe at 20, 170, and 250 K, respectively. This phenomenon is different from those reported earlier where the HSF increased monotonously with the increase of the critical temperature,10,12 and that will be discussed in detail below.
The magnetization moments after deducting the AF signal at 300 K [Fig. S3(h)†] yield a clear S-shape dependence of the applied magnetic field. Remarkably, no coercivity was found, which might betoken an appearance of superparamagnetism. In fact, from the magnifications of the central part of the M–H curves obtained at 20 K, below Tp1, [the inset of Fig. 4(b)] and 170 K, between Tp1 and Tp2, [the inset of Fig. 4(c)], it can be clearly seen that coercivity exists at 20 K while it is absent at 170 K. This suggests that Tp1 might be the particle blocking temperature (TB) of the SPM system. In order to further explore the origin of Tp1 and Tp2, ZFC magnetization curves were recorded under different magnetic fields (Fig. 5). The Tp1 (<150 K) shifts to lower temperature with the increasing magnetic field. The cusp temperature under different magnetic fields obeys the relationship Hp12 ∝ (1 − Tp1), and the curve of Tp vs. Hp [where Tp1 is defined as the maximum in Fig. 5(a)–(c)] is shown in Fig. 5(d), which is as expected for a SPM system.13–15 These results confirm the above conclusions about superparamagnetism based on the M–H curves at different temperatures. For Fig. 5(e)–(g), Tp2 is defined as the inflection point of each curve, and there is a distinct difference between the plots of Tp2 and Tp1 vs. Hp. The plateau of Tp2 at ∼ 292 K [Fig. 5(h)] is in contrast to the sharp decrease of Tp1 with the decreasing Hp. In most AF systems, the field dependence of the critical phase boundary is very small within the range of the usually accessible experimental field values.16 Therefore, we can conclude that the Tp2 is the AF phase transition temperature (TN). The TN of our AF Cr2O3 particles is reduced in comparison with the bulk value (308 K),4,9,10 which would be a result of the decrease in particle size.10,16
 | ||
| Fig. 5 Field dependence of the ZFC curves at (a and e) 5 Oe; (b and f) 1000 Oe; and (c and g) 2000 Oe. (d and h) Plots of Tp1 or Tp2 vs. Hp (black circles); red lines show the fitted results. | ||
It is common to observe hysteresis loops and divergence between ZFC/FC curves in low dimension AF materials. However, there is no recognized explanation for solving these problems until now. Different models are built for each AF system. The presence of an AF core and a ferromagnetic passivation shell was proposed during investigation of the magnetic properties of MnO nanoparticles.5 It was found that γ-Mn2O3 or Mn3O4 (depending on the particle size) would be present in the particle shell due to the surface passivation. Benitez et al. attributed the irreversible magnetization of AF Co3O4 nanowires to the wire shells which form two-dimensional diluted AF systems.6 This model also applies to AF BiFe0.8Mn0.2O3 nanoparticles.17 The picture is more complicated for NiO systems. Claims such as the presence of Ni3+ ions within the AF NiO lattice,18 AF interaction with canted surface spins,3 or surface spin disorder induced spin-glass behavior rather than SPM19 were proposed to explain the anomalous magnetic properties in NiO nanoparticles. In our system, an unexpected SPM behavior and spin-flop (SF) transition have been observed. Specifically, the SPM signals could exist above the TN of Cr2O3, which means the magnetic coupling of the SPM cannot be due to superexchange among Cr3+–O2−–Cr3+. What is the origin of superparamagnetism in AF Cr2O3 particles? How to generate net moments in this AF system? Many different mechanisms based on the origin of moments have been proposed for the oxide systems: (1) defects bear the brunt of the origin, especially O vacancies: CuO (O vacancies),20 and CoO (O vacancies);21 (2) the mixed valences could cause ferromagnetism, such as for CeO2 (the surface Ce3+/Ce4+ pairs),22 and VO2 (the valence charge defects with unpaired electrons V5+ in VO2 thin films).23 In order to further identify the origin of the moments in our system, Cr2O3–O2 samples were prepared. The M–H curve of Cr2O3–O2 is shown in Fig. S5† (red line). The hysteresis loops of the as-prepared sample and the sample after annealed in oxygen (Cr2O3–O2) are similar, which indicates that the observed SPM should not be directly related to O vacancies.
The chemical states of the compositional elements in the Cr2O3 particles were revealed using XPS. In Fig. 6, the survey spectrum, the indexed peaks only correspond to elements Cr, O, and C, where the binding energies are calibrated using the carbon C 1s peak (285.0 eV). The peak in the Cr 2p3/2 spectrum (the inset of Fig. 6) is not totally symmetrical, and can be well fitted by three peaks with different binding energy components:24 the dominant peak located at 577.2 eV is assigned to Cr3+ ions, and the other binding energy components can match Cr4+ ions (576.3 eV) and Cr6+ ions (579.0 eV). It is common to see the presence of a higher oxidation state at the surface of the transition metal oxides which have several stable oxidation states due to surface effects. For example, in epitaxial undoped VO2 thin films which are grown by pulsed laser deposition, different valence states of the V ions were found at the surface by XPS characterization.23 The major part of the surface V ions has a higher oxidation state (V5+) and makes up 47% of the V ions. Another example is AF MnO nanoparticles, the Mn ions at the particle surface are oxidized to a higher valence (Mn3+) due to the passivation effect and form a surface passivation shell of γ-Mn2O3 or Mn3O4.5 Similarly, the surface of the as-grown CoO nanoparticles appears oxidized up to Co3O4, and the surface effects here are described as a large amount of point defects which adsorb mainly oxygen species.25 Valence states of +2, +3, +4, and +6 are familiar for Cr ions, and our samples were synthesized in the air. Therefore it is possible to obtain higher oxidation states of the Cr ions than “+3” at the particle surfaces. Reddy et al. investigated the magnetic coupling of Cr2On (n = 1–6) and found that the moments at the Cr sites in Cr2O3 are antiferromagnetically coupled; the moments at the two Cr sites in CrO2 are ferromagnetically coupled (TC ≈ 396 K); while the Cr sites have negligible moments in CrO3.26 Thus, we further consider that the appearance of SPM in the Cr2O3 nanoparticles is owing to the presence of Cr4+ surface states rather than O vacancies.
 | ||
| Fig. 6 XPS survey spectrum. The inset shows a high resolution scan and fitted results of the Cr 2p3/2 core level. | ||
As discussed above, the anomalous behavior of the ZFC curve [Fig. 4(a)] could be explained by the formation of a Cr2O3 core with a CrO2 surface state system: the moments (FM CrO2 surface) of the particles are blocked below the TB (∼91 K), and SPM occurs above TB; the kinks at 292 K in the curve must be caused by the order/disorder transition of the AF Cr2O3 cores.13 Néel predicted that AF materials in fine particle form should exhibit some interesting magnetic properties including superparamagnetism and a weak ferromagnetism, which has been also observed in various AF particles as the particle size decreases. As the particle size decreases, a net magnetic moment is produced due to the nonexact compensation of the two magnetic sublattices, for example, imbalance in the number of “up and down” spins. A SPM susceptibility, due to uncompensated spins, can dominate over the AF contribution itself.11,27,28 In the synthesized Cr2O3 nanoparticles (Fig. 7), we also observed similar results: the cores show regular AF orders, whereas the surface exhibits superparamagnetism from the symmetry breaking by surface Cr4+ cations (as well, Cr6+ cations have negligible moments). These results are similar to previous studies on Co3O4 nanostructures and MnO nanoparticles, where AF systems are usually governed by core–shell behavior.5,16
The SF phenomenon as a kind of striking nonlinear effect in antiferromagnets is observed when H equals a critical field HSF given by:10,12
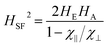 | (1) |
Furthermore, through the molecular-field constant λ and the hypothesis that χ⊥ ∼ 1/λ when HE ≫ HA, eqn (1) transforms into:10–12
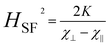 | (2) |
4 Conclusions
In summary, bimagnetic Cr2O3 nanoparticles with a corundum structure were directly synthesized by thermal decomposition. A SF transition in the AF core occurs below TN (292 K), and the appearance of a SPM surface state is owing to the valence increase (Cr4+ state). In addition, the SPM surfaces influence the inner AF cores through the exchange field, which leads to the increase of HSF below TB. We hope our work can give an insight into the magnetic properties of Cr2O3 nanoparticles and provide a path to control magnetic ordering in AF materials.Acknowledgements
This work is supported by the National Science Fund for Distinguished Young Scholars (grant nos 50925103 and 11034004), the Keygrant Project of Chinese Minisity of Education (grant no. 309027), and NSFC (grant no. 50902065).References
- B. Dieny, V. S. Speriosu, S. Metin, S. S. P. Parkin, B. A. Gurney and P. Baumgart, J. Appl. Phys., 1991, 69, 4774 CrossRef CAS PubMed.
- V. Skumryev, S. Stoyanov, Y. Zhang, G. Hadjipanayis, D. Givord and J. Nogues, Nature, 2003, 423, 850–853 CrossRef CAS PubMed.
- J. B. Yi, J. Ding, Y. P. Feng, G. W. Peng, G. M. Chow, Y. Kawazoe, B. H. Liu, J. H. Yin and S. Thongmee, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 224402 CrossRef.
- R. K. Zheng, H. Liu, R. K. Zheng, H. Liu, Y. Wang and X. X. Zhang, Appl. Phys. Lett., 2004, 84, 702–704 CrossRef CAS PubMed.
- A. López-Ortega, D. Tobia, E. Winkler, I. V. Golosovsky, G. Salazar-Alvarez, S. Estradé, M. Estrader, J. Sort, M. A. González, S. Surinach, J. Arbiol, F. Peiró, R. D. Zysler, M. D. Baró and J. Nogués, J. Am. Chem. Soc., 2010, 132, 9398–9407 CrossRef PubMed.
- M. J. Benitez, O. Petracic, E. L. Salabas, F. Radu, H. Tüysüz, F. Schüth and H. Zabel, Phys. Rev. Lett., 2008, 101, 097206 CrossRef CAS.
- S. Mandal, S. Banerjee and K. S. R. Menon, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 214420 CrossRef.
- M. Ghosh, K. Biswas, A. Sundaresan and C. N. R. Rao, J. Mater. Chem., 2006, 16, 106–111 RSC.
- M. Fiebig, D. Fröhlich and H. J. Thiele, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, R12681–R12684 CrossRef CAS.
- D. Tobia, E. Winkler, R. D. Zysler, M. Granada and H. E. Troiani, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 104412 CrossRef.
- R. D. Zysler, D. Fiorani, A. M. Testa, L. Suber, E. Agostinelli and M. Godinho, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 212408 CrossRef.
- S. Foner and S. Hou, J. Appl. Phys., 1962, 33, 1289–1290 CrossRef CAS PubMed.
- F. C. Fonseca, G. F. Goya, R. F. Jardim, R. Muccillo, N. L. V. Carreno, E. Longo and E. R. Leite, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 104406 CrossRef.
- R. K. Zheng, H. Gu, B. Xu and X. X. Zhang, J. Phys.: Condens. Matter, 2006, 18, 5905–5910 CrossRef CAS PubMed.
- S. K. Sharma, J. M. Vargas, E. D. Biasi, F. Béron, M. Knobel, K. R. Pirota, C. T. Meneses, S. Kumar, C. G. Lee, P. G. Pagliuso and C. Rettori, Nanotechnology, 2010, 21, 035602 CrossRef CAS PubMed.
- M. J. Benitez, O. Petracic, H. Tüysüz, F. Schüth and H. Zabel, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 134424 CrossRef.
- P. K. Manna, S. M. Yusuf, R. Shukla and A. K. Tyagi, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 184412 CrossRef.
- I. S. Jacobs and C. P. Bean, in Magnetism, ed. G. T. Rado and H. Suhl, Academic Press, New York, 1963, vol. III, p. 294 Search PubMed.
- S. D. Tiwari and K. P. Rajeev, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104433 CrossRef.
- D. Gao, G. Yang, J. Li, J. Zhang, J. Zhang and D. Xue, J. Phys. Chem. C, 2010, 114, 18347–18351 CAS.
- G. Yang, D. Gao, Z. Shi, Z. Zhang, J. Zhang, J. Zhang and D. Xue, J. Phys. Chem. C, 2010, 114, 21989–21993 CAS.
- M. Li, S. Ge, W. Qiao, L. Zhang, Y. Zuo and S. Yan, Appl. Phys. Lett., 2009, 94, 152511 CrossRef PubMed.
- T.-H. Yang, S. Nori, H. Zhou and J. Narayan, Appl. Phys. Lett., 2009, 95, 102506 CrossRef PubMed.
- G. Z. Xing, J. B. Yi, D. D. Wang, L. Liao, T. Yu, Z. X. Shen, C. H. A. Huan, T. C. Sum, J. Ding and T. Wu, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 174406 CrossRef.
- L. Soriano, M. Abbate, A. Fernández, A. R. González-Elipe, F. Sirotti and J. M. Sanz, J. Phys. Chem. B, 1999, 103, 6676–6679 CrossRef CAS.
- V. Reddy and S. N. Khanna, Phys. Rev. Lett., 1999, 83, 3170–3173 CrossRef.
- R. N. Bhowmik, R. Nagarajan and R. Ranganathan, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 054430 CrossRef.
- S. A. Makhlouf, J. Magn. Magn. Mater., 2004, 272–276, 1530–1532 CrossRef CAS PubMed.
- J. O. Artman, J. C. Murphy and S. Foner, Phys. Rev., 1965, 138, 342–349 CrossRef.
- T. Nagamiya, Prog. Theor. Phys., 1951, 6, 342–349 CrossRef PubMed.
- S. Foner, Phys. Rev., 1963, 130, 183–197 CrossRef CAS.
- G. N. Rao, Y. D. Yao and J. W. Chen, IEEE Trans. Magn., 2005, 41, 3409–3411 CrossRef CAS.
- S. A. Makhlouf, H. Al-Allar and R. H. Kodama, Solid State Commun., 2008, 145, 1–4 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04009d |
| This journal is © The Royal Society of Chemistry 2015 |




