Hydrogen bond effect on the photophysical properties of 2-ureido-4[1H]-pyrimidinone quadruple hydrogen bonded systems†
Jun-Sheng Chenacd,
Feng-Jiao Zhaoad,
Yang Yanga and
Tian-Shu Chu*ab
aState Key Laboratory of Molecular Reaction Dynamics, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, People's Republic of China. E-mail: tschu@dicp.ac.cn; tschu008@163.com
bInstitute for Computational Sciences and Engineering, Laboratory of New Fiber Materials and Modern Textile, The Growing Base for State Key Laboratory, Qingdao University, Qingdao, 266071, People's Republic of China
cDepartment of Chemical Physics, Lund University, 22100 Lund, Sweden
dUniversity of the Chinese Academy of Sciences, Beijing, 100049, People's Republic of China
First published on 14th April 2015
Abstract
In this work, spectroscopic techniques and quantum chemistry calculations were used to investigate the photophysical properties of 2-ureido-4[1H]-pyrimidinone (UPy) systems in two different solvents of DMSO and DCM. The investigations were carried out on the three UPy systems (AnUP, NaUP and UPNa) with two different choromophores (i.e., anthracene and naphthalene) located at the head and the tail part of the UPy module. In DCM the UPy systems exist as the Keto-2 form that self assembles into a dimer through quadruple H-B arrays AADD–DDAA (solute–solute hydrogen bonds). In DMSO the UPy systems exist in the Keto-1 form which then forms a hydrogen bond complex with the solvent through solute–solvent interaction. The differences in excited state hydrogen bond dynamics and in configuration dynamical processes, account for the measured different fluorescence lifetimes and fluorescence quantum yields (FQY, ΦF) of the UPy systems studied in this work.
1. Introduction
The hydrogen-bond (H-B) plays an essential role in physics, chemistry, biology and material sciences.1 Nature makes use of the “site-specific” H-B interaction to assemble relatively simple molecular precursors into extremely complex biomolecules, such as DNA, proteins and hydrogen bound networks, which are vital for life processes. Scientists employ the H-B interaction to construct H-B crystal engineering, functional polymers and self-assembled supramolecular architectures, which can be used as materials with a specific functionality. Due to the importance of the widely used H-B interaction, the properties of the ground and excited state H-B have attracted broad attention.Meijer and co-workers2 have previously developed the 2-ureido-4[1H]-pyrimidinone (UPy) AADD (A = acceptor of the H-B, D = donor of the H-B) binding module with high dimerization constants. In order to study the dimerization of UPy, they measured the 1H NMR spectra in CDCl3 and in mixture of CDCl3 with DMSO-d6.2 The results showed that in CDCl3 the dimerization constants can exceed 106 M−1 and in CDCl3–DMSO-d6 mixture solution the Keto-1 form and Keto-2 are in equilibrium with each other.2 This technique reported in ref. 2 is helpful for the confirmation of structure and purity and can be used to quantify the different tautomers for the UPy system. Since then, the quadruple H-B module has been extensively applied in supramolecular oligomers and polymers.3–8 For example, Meijer and co-workers4 employed the UPy AADD binding module to connect three different π-conjugated oligomers and developed a white emissive supramolecular polymer, which showed high solution viscosities and tunable emission colors.4 The white light emission of the polymer is based on the excited state energy transfer process between different π-conjugated oligomers though the quadruple H-B module.4 Due to the importance of the excited state energy transfer for the white light emission of the polymer, a lot of models based on UPy quadruple H-B have been developed to investigate the singlet–singlet/triplet–triplet energy transfer (ET),7,9–13 photo-induced electron transfer (PET)14–16 and intramolecular charge transfer (ICT)17 processes. These researches demonstrated that the quadruple H-B plays an important role for energy, or electron, transfer channel in the excited state.
In the electronically excited state, H-B can influence the excited state dynamics processes involving ET, PET, ICT, excited state proton transfer and so on.1,18,19 In recent years, the electronic excited-state H-B has been widely investigated with powerful experimental methods, such as time-resolved infrared vibrational spectroscopy, femtosecond fluorescence upconversion and transient absorption spectra, and state-of-the-art theoretical methods.20–27 Han and co-workers1,28–32 have theoretically and experimentally investigated the excited-state intermolecular H-B dynamics and demonstrated the significant role of the electronic excited-state H-B on photophysical processes including PET, ICT and metal-to-ligand charge transfer. In particular, they are the first ones to suggest the PET mechanism facilitated by H-B for fluorescence quenching,31,32 which has been widely used in explaining sensing mechanism for various fluorescent probes; they also provided a rule where the intermolecular H-B strengthening/weakening corresponds to red-shifts/blue-shifts in the electronic spectra.33 Domcke and Sobolewski34–37 studied the role of intramolecular H-B effects on the photophysical properties of many intramolecular H-B aromatic systems with quantum chemistry calculation methods.
In the condense phase there are three kinds of H-Bs: two kinds of intermolecular H-B (solvent–solute H-B and solute–solute H-B) and one kind of intramolecular H-B. Different H-Bs have different effects on the photophysical and photochemical properties of chromophores, their excited state dynamics process are different as well. As previously reported, three different tautomers (Keto-1, Keto-2 and Enol, see Fig. 1) of UPy are present in solution, and the tautomeric equilibrium constants are influenced by the polarity of solvent and are concentration-dependent.38 The o-quinonoid (Keto-1) form, a 4[3H]-pyrimidinone (or 6[1H]-pyrimidinone), is predominant in certain media38,39 (for example in dimethylsulfoxide, DMSO).15 This tautomer cannot undergo dimer self-assembly through quadruple H-B. In DMSO, it can interact with DMSO through solute–solvent intermolecular H-B. The Keto-2 form, 4[1H]-pyrimidinone, and the Enol form, 4[1H]-pyrimidinol, can undergo dimer self-assembly through quadruple H-B arrays AADD–DDAA and DADA–ADAD, respectively.38 Generally the tautomer Keto-2 is favored in nonpolar solvents (for example in dichloromethane, DCM).12,13,15,38,39 In DCM, Keto-2 can undergo dimerization through solute–solute H-B. An intramolecular H-B can form in the three kinds of tautomer and thus further stabilize the dimers.38,40
 | ||
| Fig. 1 Tautomer and monomer–dimer equilibrium in UPy system, and molecular structures of AnUP, NaUP and UPNa. | ||
In the present work we focus specifically on a suite of compounds containing UPy module to determine the effects of intermolecular H-B (solute–solvent H-B and solute–solute H-B) and intramolecular H-B on the electronic transitions and photophysical properties. Spectroscopic and theoretical investigations were employed on three unique species including 6-methyl-5-(9-methylene-anthracene)-(2-butylureido-4[1H]-pyrimidinone) (AnUP),12,15 2-(2-naphthalene)ureido-6-(1-undecyl)-4[1H]-pyrimidino-ne (NaUP)12 and 1-(naphthalen-2-yl)-3-(4-oxo-6-undecyl-1,4-dihydropyrimidin-2-yl)urea (UPNa).13 A comparison of the steady-state absorption and fluorescence spectra, and fluorescence lifetimes of these compounds in DMSO and DCM allow insight into the effects of different H-B. Furthermore, we discussed along with relevant DFT and TDDFT results, which provide information about the nature of the low-lying excited states of these compounds.
2. Experimental and theoretical methods
The compounds AnUP, NaUP and UPNa (see Fig. 1) were prepared according to literature procedures.12,13,15 In order to obtain the Keto-1 and Keto-2 forms, all absorption and emission experiments were conducted in dichloromethane (DCM; CH2Cl2) and dimethyl sulfoxide [DMSO, (CH3)2SO], respectively. All solvents used were high-performance liquid chromatography grade (super dry solvent purchased from J&K Chemical).UV-Vis absorption spectra were obtained using a PerkinElmer Lambda35 spectrophotometer. The steady-state fluorescence, and time-resolved fluorescence decays were recorded using a Horiba JobinYvon FluoroMax-4 spectrofluorometer. Time-resolved fluorescence decays were recorded using the time-correlated single photon counting method. Data analysis was conducted using commercial software provided by Horiba Instruments.
All calculations presented were accomplished using the DFT/TDDFT method with the long-range corrected hybrid density functional wB97XD41 and SVP basis set42,43 by Gaussian 09 program.44 Considering that the experiments were conducted in DCM and DMSO, in all the calculations solvent effects were included by using the integral equation formalism45,46 (IEF) version of polarizable continuum model47,48 (PCM) with the dielectric constant of DCM (ε = 8.9) and DMSO (ε = 46.8). For the ground state (S0) structures, we optimized these structures without constrictions. Vertical excitation energies (VEE) calculations were performed from the ground optimized structure using TDDFT/wB97XD/SVP method with IEF-PCM (DCM, ε = 8.9; DMSO, ε = 46.8). For the first excited state, the geometries were optimized from the optimized ground sate structures without constraints and studied using the TDDFT/wB97XD/SVP method with IEF-PCM (DCM, ε = 8.9; DMSO, ε = 46.8).
3. Results and discussion
3.1 Steady-state absorption and emission
Fig. 2 shows the steady-state absorption and emission spectra of AnUP, NaUP and UPNa in two different solvents (DCM and DMSO, the details of the measured absorption and fluorescence emission are listed in Table S1†). The absorption and emission spectra of AnUP12,15 and the absorption spectrum of UPNa13 have been reported, and our measurements agree well with previously reported results. In DMSO the absorption spectra of AnUP, NaUP and UPNa are red-shifted compared with those in DCM. AnUP in DMSO, the absorption coefficient is increased (at around 300 nm). [see Fig. 2(a)] As shown in Fig. 1 for UPy system, the Keto-1 form is predominant in DMSO, and the Keto-2 is predominant in DCM.15,38 The different tautomer structures have different electronic states and result in the enhanced absorption band around 300 nm. In DMSO, the fluorescence emission spectra of these UPy compounds are red-shifted compared with those in DCM. The red-shift can be ascribed to the combination of the relatively strong polarity of DMSO and the different tautomer structures of UPy compounds in DMSO and DCM. And it also reflects an overall strengthening trend of the H-B in the DMSO solution.33 In DMSO the Keto-1 form can interact with solvent by H-B. As shown in Fig. 1 two H-B donors can form solute–solvent hydrogen bonds between the UPy compounds and DMSO. In DCM the Keto-2 form can form solute–solute H-B and undergo dimerization through quadruple H-B arrays AADD–DDAA. In Keto-1 and Keto-2 tautomer structures, the different H-Bs can induce the red-shifted spectrum. One can notice that the absorption spectrum of AnUP shows a vibronic progression. This phenomenon is ascribed to the π–π* transition of anthracene moiety in this compounds. This vibronic progression is still kept in the fluorescence emission spectrum of AnUP.3.2 Time-resolved fluorescence spectra
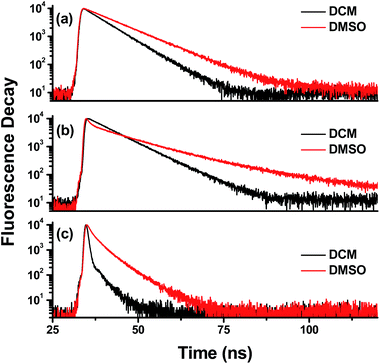
The time-resolved fluorescence decays of AnUP, NaUP and UPNa at excitation wavelength of 295 or 376 nm under different emission wavelengths in DCM and DMSO were measured. Fig. 3 shows the time-resolved fluorescence decays for UPy compounds in DCM and DMSO. The fluorescence decay process was fitted by single, double, or triple exponential. Fluorescence quantum yield (FQY, ΦF), fluorescence lifetime values, the corresponding relative amplitudes (RAs) and their average lifetime values are listed in Tables 1–3 for AnUP, NaUP and UPNa, respectively.| Solvent | λemis/nm | FQY (%) | τ/ns | RA | χ2 |
|---|---|---|---|---|---|
| DCM | 415 | 54.7 ± 0.2 | 6.5 ± 0.01 | 100 | 1.274 |
| DMSO | 418 | 77.8 ± 0.2 | 9.5 ± 0.01 | 100 | 1.102 |
| Solvent | λemis/nm | FQY | τ1 | RA1 | τ2 | RA2 | τ3 | RA3 | τa | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| DCM | 334 | 2.02 ± 0.01 | 8.0 ± 0.01 | 100 | — | — | — | — | 8.0 ± 0.01 | 1.211 |
| DMSO | 370 | 0.34 ± 0.04 | 0.64 ± 0.01 | 9.74 | 11.4 ± 0.07 | 65.44 | 27.6 ± 0.16 | 24.82 | 14.4 ± 0.09 | 1.169 |
| Solvent | λemis/nm | FQY | τ1 | RA1 | τ2 | RA2 | τ3 | RA3 | τa | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| DCM | 353 | 0.43 ± 0.01 | 0.2 ± 0.004 | 92.56 | 3.5 ± 0.05 | 7.44 | — | — | 0.5 ± 0.01 | 1.186 |
| DMSO | 370 | 2.19 ± 0.26 | 0.4 ± 0.01 | 20.60 | 2.4 ± 0.05 | 44.32 | 5.9 ± 0.03 | 35.08 | 3.2 ± 0.04 | 1.195 |
As listed in Table 1 the fluorescence lifetime of AnUP in DMSO (τ = 9.5 ns) is longer than that in DCM (τ = 6.5 ns), here the measured lifetime in DCM is consistent with previously reported value 6.5 ns (ref. 15), corresponding to the FQY of AnUP in DMSO (FQY = 77.8%) is higher than that in DCM (FQY = 54.7%). [here the FQY for AnUP is collected relative to anthracene (ΦF = 27% in ethanol).] This indicates that, in DMSO, the Keto-1 form of AnUP has relatively longer fluorescence lifetime and higher FQY. Thus, the solute–solvent hydrogen bond between AnUP and DMSO can stabilize the fluorescence excited state of AnUP and enhance the fluorescence radiation transition process.
For NaUP and UPNa, the chromophore naphthalene is located at the head and the tail part of NaUP and UPNa, respectively. As listed in Table 2, the FQY of NaUP in DMSO (ΦF = 0.34%) is lower than that in DCM (ΦF = 2.02%). [here the FQY for NaUP and UPNa is collected relative to naphthalene (ΦF = 20.5% in ethanol)] While the fluorescence lifetime of NaUP in DMSO (14.4 ns) is longer than that in DCM (8.0 ns), here the measured lifetime in DCM is consistent with previously reported value 8.2 ns (ref. 12). The fluorescence decay of NaUP in DMSO is fitted by triple exponential function. For UPNa, the FQY in DMSO (ΦF = 2.19%) is relatively higher than that in DCM (ΦF = 0.43%). The average fluorescence lifetime of UPNa in DMSO (3.2 ns) is relatively longer than that in DCM (0.5 ns). This tendency between FQY and fluorescence lifetime agrees with that in AnUP. The fluorescence lifetime of UPNa in DCM is fitted by double exponential function, but the relative amplitude of τ2 = 3.5 ns is only 7.44%. For UPNa in DMSO, the fluorescence lifetime is fitted by triple exponential function. For NaUP and UPNa, the different fluorescence decay behaviors in DCM and DMSO indicate that the different types of H-Bs (solute–solute H-B and solvent–solute H-B) significantly affect the excited state dynamics of NaUP and UPNa. The multiple exponential fluorescence decay is attributable to the different decay processes of the fluorescence emission from the different local minimal configurations on the S1 excited state. Though Keto-1 is predominant and is favored in DMSO based on the previously reported studies,12,13,15,38,39 there still has some opportunity for the presence of the other two different tautomers in DMSO (see Fig. 1, Keto-2 and Enol tautomers). Therefore, these different tautomers can show different fluorescence decay processes.
3.3 DFT and TDDFT calculations
The optimized configurations of AnUP, NaUP and UPNa in DCM and DMSO are shown in Fig. 4. In DCM these UPy systems can undergo dimerization through quadruple H-B arrays AADD–DDAA, in DMSO they can interact with DMSO molecules by solute–solvent interaction and form complexes through H-Bs. The lengths and angles of hydrogen bonds are listed in Tables S2–S4† for AnUP, NaUP and UPNa, respectively. For AnUP in DCM, the AnUP dimer is stable. In AnUP-dimer the calculated values of H-B length agree well with the previously theoretical reported results.38,49 Meanwhile, the two outer hydrogen bonds (O1′⋯H1 and O1⋯H1′) are 0.21 Å shorter than the two inner H-Bs (N3′⋯H2 and N3⋯H2′), which is in good agreement with the previously reported X-ray data and theoretical results (0.21 Å).15,49 The calculated values of H-B angle agree well with the previously reported X-ray data and theoretical results as well.15,49 These agreements between this calculation results and previously reported experimental X-ray data and theoretical results indicate that our calculation is reliable. As shown in Fig. 4 Keto-1 form of these UPy systems have N–H moieties which act as H-B donor to form stable H-B with two DMSO molecules. The ground state optimized structures demonstrate that the solute–solute and solute–solvent H-Bs exist in these UPy systems. The dimerization binding energies in DCM and binding energies (EB) between UPy systems and DMSO molecules have been calculated and listed in Table 4. The basis set superposition error (BSSE) has been accounted for using the counterpoise method of Boys and Bernardi.50 Here, the calculated binding energies can explain that the quadruple H-B arrays AADD–DDAA are extremely stable in DCM. And in DMSO the UPy molecules can interact with DMSO molecules through strong H-Bs. One can notice that the binding energies of UPNa (EB = 60.2 kcal mol−1) is higher than that of AnUP (EB = 56.2 kcal mol−1) and NaUP (EB = 57.6 kcal mol−1), respectively. This is due to the chromophore being located in different parts of these UPy systems. That is, for UPNa, chromophore is located at tail part while for AnUP and NaUP, chromophore is located at head part. The electron rich nature of chromophore can enhance the strength of H-B, and this effect will be more apparent when the chromophore is close to the H-B. On the other hand, the different position of the chromophores also influences the tautomeric equilibria2 as can be seen in Fig. 1. Based on the previously reported studies12,13,15,38,39 we can confirm that for these UPy systems, Keto-2 is favored in CH2Cl2 and Keto-1 is favored in DMSO. Further, the calculated different binding energies indicate that the position of the chromophore can influence the dimerization constants. | ||
| Fig. 4 Optimized structures for AnUP, NaUP and UPNa dimers (in DCM) and monomer complexes (in DMSO). Gray: C; red: O; blue: N; yellow: S; white: H. | ||
| UPy systems | AnUP–DCM | AnUP–DMSO | NaUP–DCM | NaUP–DMSO | UPNa–DCM | UPNa–DMSO |
|---|---|---|---|---|---|---|
| Binding energy/kcal mol−1 | 56.2 | 26.4 | 57.6 | 26.1 | 60.2 | 27.3 |
The calculated electronic transition energies and the corresponding oscillator strengths (f) of the first singlet excited state transition (S0 → S1) for UPy systems in DCM and DMSO are listed in Tables 5 and 6, respectively, which show that the calculated lowest vertical excitation energies for AnUP, NaUP and UPNa are at 354, 268, and 281 nm in DCM, and at 354, 269, and 284 nm in DMSO, respectively, consistent with the experimental results discussed previously. The calculated frontier molecular orbitals involved in the S0 → S1 electronic transition for AnUP, NaUP and UPNa in DCM and DMSO are shown in Fig. 5. For the S0 → S1 electronic transition of AnUP in DCM and DMSO, the corresponding orbitals with π-type symmetry are mainly localized on the anthracene moiety. Clearly, the first singlet transition of NaUP and UPNa in DCM has charge-separation character. The charge-separation character of UPNa in DCM (S0 → S1 transition) is more pronounced compared with that of NaUP. The first singlet transition of NaUP in DMSO possesses intramolecular charge transfer character (form pyrimidine moiety to naphthalene, see HOMO → LUMO and HOMO−1 → LUMO+2 in Fig. 5) and intermolecular charge transfer character (from solvent molecule DMSO to NaUP molecule, see HOMO−3 → LUMO in Fig. 5). The first singlet transition of UPNa in DMSO possesses intramolecular charge transfer character (form naphthalene to pyrimidine moiety, see HOMO → LUMO+1 in Fig. 5).
| Electronic transitiona | Energy (nm eV−1) | fb | Contrib.c | CId | |
|---|---|---|---|---|---|
| a Only the first excited state transition S0 → S1 is presented.b Oscillator strength.c Only the main configurations are presented; H, HOMO (highest occupied molecular orbital) and L, LUMO (lowest unoccupied molecular orbital).d The CI coefficients are in absolute values. | |||||
| AnUP in DCM | S0 → S1 | 354 (3.50) | 0.3803 | H−1 → L | 0.47 |
| H → L+1 | 0.47 | ||||
| NaUP in DCM | S0 → S1 | 268 (4.63) | 0.0335 | H−5 → L | 0.26 |
| H−1 → L | 0.17 | ||||
| H−1 → L+5 | 0.15 | ||||
| UPNa in DCM | S0 → S1 | 281 (4.41) | 0.2324 | H−1 → L | 0.28 |
| H → L+1 | 0.28 | ||||
| H → L+3 | 0.10 | ||||
| Electronic transitiona | Energy (nm eV−1) | fb | Contrib.c | CId | |
|---|---|---|---|---|---|
| a Only the first excited state transition S0 → S1 is presented.b Oscillator strength.c Only the main configurations are presented; H, HOMO (highest occupied molecular orbital) and L, LUMO (lowest unoccupied molecular orbital).d The CI coefficients are in absolute values. | |||||
| AnUP in DMSO | S0 → S1 | 354 (3.50) | 0.1917 | H → L | 0.98 |
| NaUP in DMSO | S0 → S1 | 269 (4.61) | 0.0589 | H−3 → L | 0.19 |
| H−1 → L+2 | 0.27 | ||||
| H → L | 0.21 | ||||
| UPNa in DMSO | S0 → S1 | 284 (4.37) | 0.1180 | H → L | 0.58 |
| H → L+1 | 0.15 | ||||
| H−2 → L | 0.11 | ||||
 | ||
| Fig. 5 The calculated frontier molecular orbitals involved in the S0 → S1 electronic transition for AnUP, NaUP and UPNa in DCM and DMSO. | ||
In order to further understand the excited state dynamics processes and the excited state H-Bs, the optimized structures of S1 state for AnUP, NaUP and UPNa in DCM and DMSO are obtained and showed in Fig. S2–S4,† respectively. The length of important bonds, bond angles and dihedral angles are listed in Tables S2–S4.† The configurations of AnUP in both DCM and DMSO, and NaUP in DMSO keep the similar structure in the first excited state with that in the ground state, without any evident configuration changes. For NaUP in DCM, the chromophore naphthalene is rotating in the first excited state, this can be revealed by monitoring the values of dihedral angles C5–C6–C7–C8, C5–C6–C8–C9, C5′–C6′–C7′–C8′ and C5′–C6′–C8′–C9′. For UPNa, as listed in Table S4† the chromophore (naphthalene) is rotating at the first excited state in DCM and DMSO. The intermolecular solute–solute H-Bs are strengthened in DCM. In DMSO, the intermolecular solute–solvent H-Bs are strengthened and the intramolecular H-B is weakened.
For AnUP system in DCM and DMSO, the first singlet transition of AnUP could be regarded as π–π* transition, and the configuration has no comparable changes between S1 and S0. The Keto-1 form of AnUP in DMSO has a relatively higher FQY and longer fluorescence lifetime compared to that of the Keto-2 form of AnUP in DCM. For NaUP system in DCM and DMSO, the first singlet transitions of them possess charge transfer character. In DCM the chromophore (naphthalene) of NaUP is rotating in the first excited state. In DMSO the NaUP–DMSO complex keeps a similar structure in the first excited state compared with that in the ground state. The first singlet excited state of NaUP–DMSO complex shows intermolecular charge transfer between DMSO and NaUP. The fluorescence can be quenched by the intermolecular charge transfer process between solute and solvent though H-Bs as proposed by Han and Zhao.32,51 Hence the FQY of NaUP in DMSO is relatively lower than that in DCM. For UPNa both in DCM and DMSO the S1 of UPNa shows intramolecular charge transfer, the intermolecular hydrogen bonds are strengthened, the intramolecular hydrogen bonds are weakened and the chromophore (naphthalene) is rotating in the first excited state. (see Table S4†). The synergetic effect of excited state configuration change (excited state hydrogen bond dynamic process and chromophore rotating) and charge transfer process lowers the FQY of NaUP and UPNa compared to that of AnUP.
4. Conclusion
Two different types of H-Bs (solute–solute H-B and solute–solvent H-B) have different effects on the photophysical properties of UPy systems (AnUP, NaUP and UPNa). In DMSO Keto-1 form of UPy system is the prevalent tautomer, which can form solute–solvent H-Bs between UPy systems (AnUP, NaUP and UPNa) and DMSO molecules. In DCM the Keto-2 form of UPy system is the prevalent tautomer, which can undergo dimerization (AnUP-dimer, NaUP-dimer and UPNa-dimer) through quadruple solute–solute H-B arrays AADD–DDAA. In DMSO, the Keto-1 forms of UPy systems show a slightly red-shift in both the absorption and emission spectra relative to those Keto-2 forms in DCM. The FQY of AnUP in DMSO is higher than that in DCM, as indicated by that the fluorescence lifetime of AnUP in DMSO is longer than that in DCM.The first singlet translations of AnUP in DCM and DMSO could be regarded as π–π* transition and the first excited state of AnUP keeps the similar structure as that in the ground state. For NaUP in DCM, the chromophore naphthalene is rotating in the first excited state, and the twisted charge transfer state of NaUP induces a lowering of FQY. While for NaUP in DMSO, the first excited state possesses intermolecular charge transfer character from DMSO to NaUP molecule. This process induces the NaUP in DMSO to exhibit even lower FQY. For UPNa, in the first excited state, the intermolecular H-Bs are strengthened and the intramolecular H-Bs are weakened both in DCM and DMSO. In comparison, the chromophore (anthracene) is located at the head part of AnUP, and the chromophore (naphthalene) is located at the head and the tail part NaUP and UPNa, respectively. The photophysical properties of UPy can be modulated by the location of chromophore, due to the chromophore influencing the strength of intermolecular H-Bs. For different chromophores the photophysical properties of UPy system can be affected by solute–solute or solute–solvent intermolecular hydrogen bond in different ways.
Acknowledgements
This work is supported by the National Basic Research Program of China (2013CB834604), the National Natural Science Foundation of China (NSFC grant nos 21403226 and 21273234) and the Shandong Provincial Natural Science Foundation of China (ZR2014AM025). Thanks for Professor Li-Zhu Wu for her supply of the samples. We thank Dr Yizhu Liu for helped with the 1H NMR measurement and Mr Alexander Christea for the help with editing the paper.References
- G. J. Zhao and K. L. Han, Acc. Chem. Res., 2012, 45, 404–413 CrossRef CAS PubMed.
- F. H. Beijer, R. P. Sijbesma, H. Kooijman, A. L. Spek and E. W. Meijer, J. Am. Chem. Soc., 1998, 120, 6761–6769 CrossRef CAS.
- R. P. Soojbesma, M. H. P. V. Genderen and E. W. Meijer, J. Am. Chem. Soc., 2000, 122, 7487–7493 CrossRef.
- R. Abbel, C. Grenier, M. J. Pouderoijen, J. W. Stouwdam, P. E. L. G. Leclère, R. P. Sijbesma, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2009, 131, 833–843 CrossRef CAS PubMed.
- H. M. Keizer, J. J. Gonzalez, M. Segura, P. Prados, R. P. Sijbesma, E. W. Meijer and J. de Mendoza, Chem.–Eur. J., 2005, 11, 4602–4608 CrossRef CAS PubMed.
- U. Hahn, J. J. Gonzalez, E. Huerta, M. Segura, J. F. Eckert, F. Cardinali, J. de Mendoza and J. F. Nierengarten, Chem.–Eur. J., 2005, 11, 6666–6672 CrossRef CAS PubMed.
- S. P. Dudek, M. Pouderoijen, R. Abbel, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2005, 127, 11763–11768 CrossRef CAS PubMed.
- B. Sridhar and K. Ravikumar, Angew. Chem., Int. Ed., 2001, 66, o414–417 Search PubMed.
- E. E. Neuteboom, E. H. A. Beckers, S. C. J. Meskers, E. W. Meijer and R. A. J. Janssen, Org. Biomol. Chem., 2003, 1, 198–203 CAS.
- E. H. A. Beckers, P. A. van Hal, A. Schenning, A. El-ghayoury, E. Peeters, M. T. Rispens, J. C. Hummelen, E. W. Meijer and R. A. J. Janssen, J. Mater. Chem., 2002, 12, 2054–2060 RSC.
- F. J. M. Hoeben, L. M. Herz, C. Daniel, P. Jonkheijm, A. P. H. J. Schenning, C. Silva, S. C. J. Meskers, D. Beljonne, R. T. Phillips, R. H. Friend and E. W. Meijer, Angew. Chem., Int. Ed., 2004, 43, 1976–1979 CrossRef CAS PubMed.
- C. C. Zhao, Q. X. Tong, Z. T. Li, L. Z. Wu, L. P. Zhang and C. H. Tung, Tetrahedron Lett., 2004, 45, 6807–6811 CrossRef CAS.
- S.-M. Wang, M.-L. Yu, J. Ding, C.-H. Tung and L.-Z. Wu, J. Phys. Chem. A, 2008, 112, 3865–3869 CrossRef CAS PubMed.
- A. P. H. J. Schenning, J. V. Herrikhuyzen, P. Jonkheijm, Z. Chen, F. Würthner and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 10252–10253 CrossRef CAS PubMed.
- Y. P. Zhao, C. C. Zhao, L. Z. Wu, L. P. Zhang, C. H. Tung and Y. J. Pan, J. Org. Chem., 2006, 71, 2143–2146 CrossRef CAS PubMed.
- M. L. Yu, S. M. Wang, K. Feng, T. Khoury, M. J. Crossley, Y. Fan, J. P. Zhang, C. H. Tung and L. Z. Wu, J. Phys. Chem. C, 2011, 115, 23634–23641 CAS.
- C. C. Zhao, Z. T. Li, L. Z. Wu, L. P. Zhang and C. H. Tung, Chin. J. Chem., 2004, 22, 1391–1394 CrossRef CAS.
- A. K. Srivastava and N. Misra, Commun. Comput. Chem., 2013, 1, 328–341 Search PubMed.
- Y. H. Liu and S. C. Lan, Commun. Comput. Chem., 2013, 1, 235–243 Search PubMed.
- B. C. Westlake, M. K. Brennaman, J. J. Concepcion, J. J. Paul, S. E. Bettis, S. D. Hampton, S. A. Miller, N. V. Lebedeva, M. D. E. Forbes, A. M. Moran, T. J. Meyer and J. M. Papanikolas, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8554–8558 CrossRef CAS PubMed.
- N. Barman, D. Singha and K. Sahu, J. Phys. Chem. A, 2013, 117, 3945–3953 CrossRef CAS PubMed.
- M. C. Capello, M. Broquier, C. Dedonder-Lardeux, C. Jouvet and G. A. Pino, J. Chem. Phys., 2013, 138, 054304 CrossRef PubMed.
- K. Chevalier, M. M. N. Wolf, A. Funk, M. Andres, M. Gerhards and R. Diller, Phys. Chem. Chem. Phys., 2012, 14, 15007–15020 RSC.
- E. Krystkowiak, J. Koput and A. Maciejewski, Phys. Chem. Chem. Phys., 2012, 14, 8842–8851 RSC.
- O. A. Sytina, I. H. M. van Stokkum, D. J. Heyes, C. N. Hunter, R. van Grondelle and M. L. Groot, J. Phys. Chem. B, 2010, 114, 4335–4344 CrossRef CAS PubMed.
- L. Valle, F. E. Moran Vieyra and C. D. Borsarelli, Photochem. Photobiol. Sci., 2012, 11, 1051–1061 CAS.
- W. J. Schreier, I. Pugliesi, F. O. Koller, T. E. Schrader, W. Zinth, M. Braun, S. Kacprzak, S. Weber, W. Roemisch-Margl, A. Bacher, B. Illarionov and M. Fischer, J. Phys. Chem. B, 2011, 115, 3689–3697 CrossRef CAS PubMed.
- S. Chai, G.-J. Zhao, P. Song, S.-Q. Yang, J.-Y. Liu and K.-L. Han, Phys. Chem. Chem. Phys., 2009, 11, 4385–4390 RSC.
- G. J. Zhao and K. L. Han, J. Phys. Chem. A, 2007, 111, 9218–9223 CrossRef CAS PubMed.
- G. J. Zhao and K. L. Han, Biophys. J., 2008, 94, 38–46 CrossRef CAS PubMed.
- G.-J. Zhao, B. H. Northrop, K.-L. Han and P. J. Stang, J. Phys. Chem. A, 2010, 114, 9007–9013 CrossRef CAS PubMed.
- G. J. Zhao, J. Y. Liu, L. C. Zhou and K. L. Han, J. Phys. Chem. B, 2007, 111, 8940–8945 CrossRef CAS PubMed.
- G.-J. Zhao and K.-L. Han, ChemPhysChem, 2008, 9, 1842–1846 CrossRef CAS PubMed.
- Z. Lan, L. M. Frutos, A. L. Sobolewski and W. Domcke, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12707–12712 CrossRef CAS PubMed.
- A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2007, 111, 11725–11735 CrossRef CAS PubMed.
- L. M. Frutos, A. Markmann, A. L. Sobolewski and W. Domcke, J. Phys. Chem. B, 2007, 111, 6110–6112 CrossRef CAS PubMed.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2006, 8, 3410–3417 RSC.
- H. Sun, H. H. Lee, I. Blakey, B. Dargaville, T. V. Chirila, A. K. Whittaker and S. C. Smith, J. Phys. Chem. B, 2011, 115, 11053–11062 CrossRef CAS PubMed.
- S. M. Wang, L. Z. Wu, L. P. Zhang and C. H. Tung, Chin. Sci. Bull., 2006, 51, 129–138 CrossRef CAS.
- R. P. Sijbesma and E. W. Meijer, Chem. Commun., 2003, 6, 5–16 RSC.
- J. D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 084106 CrossRef PubMed.
- A. Schafer, H. Horn and R. Ahlrichs, J. Chem. Phys., 1992, 97, 2571–2577 CrossRef.
- A. Schafer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- E. Cances, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041 CrossRef CAS.
- B. Mennucci, E. Cances and J. Tomasi, J. Phys. Chem. B, 1997, 101, 10506–10517 CrossRef CAS.
- S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef CAS.
- R. Cammi and J. Tomasi, J. Comput. Chem., 1995, 16, 1449–1458 CrossRef CAS.
- J. S. Chen, P. W. Zhou, G. Y. Li, T. S. Chu and G. Z. He, J. Phys. Chem. B, 2013, 117, 5212–5221 CrossRef CAS PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 2002, 100, 65–73 CrossRef.
- G. J. Zhao and K. L. Han, J. Phys. Chem. A, 2007, 111, 2469–2474 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Summary of steady-state absorption and fluorescence emission measurements; calculated important bond lengths (in Å) and bond angles (in degree) for the fully optimized structures of AnUP-dimer, NaUP-dimer and UPNa-dimer (in DCM) and AnUP–DMSO complex, NaUP–DMSO complex and UPNa–DMSO complex (in DMSO) in ground state (S0) and first excited state (S1); optimized first excited state (S1) structures for AnUP-dimer, NaUP-dimer and UPNa-dimer (in DCM) and AnUP–DMSO complex, NaUP–DMSO complex and UPNa–DMSO complex (in DMSO); and the 1H NMR of AnUP, NaUP and UPNa can be consulted in the ESI. See DOI: 10.1039/c5ra04005a |
| This journal is © The Royal Society of Chemistry 2015 |