Aerobic oxidation of alcohols and alkenes over a novel lacunary phosphomolybdate anchored to zeolite Hβ†
Abstract
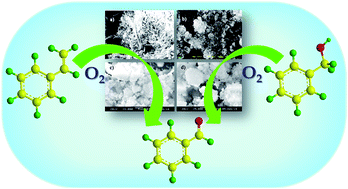
Lacunary phosphomolybdate anchored to zeolite Hβ was synthesized and characterised by various physicochemical techniques. The catalytic activity was evaluated for aerobic oxidation of benzyl alcohol and styrene. The catalyst was found to be efficient, especially in terms of selectivity for the desired benzaldehyde product (for benzyl alcohol – 90%, styrene – 72%), very high turnover number (alcohols > 3500, alkenes > 18 000) as well as recyclability. The viability of the catalyst was also extended to various alkenes and alcohols.

 Please wait while we load your content...
Please wait while we load your content...