Convenient synthesis of 2,3-disubstituted quinazolin-4(3H)-ones and 2-styryl-3-substituted quinazolin-4(3H)-ones: applications towards the synthesis of drugs†
Abstract
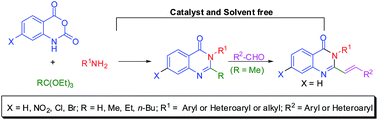
Simple, convenient, and green synthetic protocols have been developed for the one pot synthesis of 2,3-disubstituted quinazolin-4(3H)-ones and 2-styryl-3-substituted quinazolin-4(3H)-ones under catalyst and solvent free conditions. The multicomponent reaction (3-MCR) involving isatoic anhydride, an amine, and orthoester afforded the 2,3-disubstituted quinazolin-4(3H)-ones in excellent yields under classical heating at 120 °C for 5 h or under microwave irradiation at 140 °C for 20–30 min. The use of ammonium acetate instead of the amine provides the 2-substituted quinazolin-4(3H)-ones. The reactions are compatible with various substituted isatoic anhydrides, aryl/heteroaryl/alkyl/cycloalkyl amines, and orthoesters. The strategies are extended to the one pot tandem condensation involving isatoic anhydride, an amine, orthoester, and aldehyde to afford highly functionalized (E)-3-aryl/heteroaryl-2-styrylquinazolin/(2-(heteroaryl)vinyl)quinazolin-4(3H)-ones. The applications of the methodologies are demonstrated through the synthesis of various drugs which act on the central nervous system such as methaqualone, mebroqualone, mecloqualone, piriquialone, and diproqualone.

 Please wait while we load your content...
Please wait while we load your content...